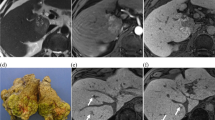
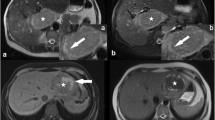
Abstract
Gadoxetic acid-enhanced magnetic resonance imaging (MRI) plays important roles in diagnosis of hepatic lesions because of its superiority in the detectability of small lesions, its differentiation ability, and its utility for the early diagnosis of hepatocellular carcinoma (HCC). In HCC, expression of organic anion transporting polypeptide (OATP) 1B3 correlates with the enhancement ratio in the hepatobiliary phase. Gadoxetic acid-enhanced MRI, an indirect molecular imaging method, reflects OATP1B3 expression in HCC. OATP1B3 expression gradually decreases from the dysplastic nodule stage to advanced HCC. Decreased expression is a sensitive marker of multistep hepatocarcinogenesis, especially in the early stages. Hypervascular HCCs commonly show hypointensity in the hepatobiliary phase corresponding to a decrease in OATP1B3; however, approximately 10% of HCCs show hyperintensity due to OATP1B3 overexpression. This hyperintense HCC shows less aggressive biological features and has a better prognosis than hypointense HCC. Hyperintense HCC can be classified into a genetic subtype of HCC with a mature hepatocyte-like molecular expression. OATP1B3 expression and the less aggressive nature of hyperintense HCC are regulated by the molecular interaction of β-catenin signaling and hepatocyte nuclear factor 4α, a tumor suppressor factor. Gadoxetic acid-enhanced MR imaging has the potential to be an imaging biomarker for HCC.
Key Points
• The hepatobiliary phase is a sensitive indirect indicator of organic anion transporting polypeptide1B3 (OATP1B3) expression in hepatocellular carcinoma (HCC).
• The OATP1B3 expression, namely, enhancement in the hepatobiliary phase, decreases from the very early stage of hepatocarcinogenesis, contributing to early diagnosis of HCC.
• HCC showing hyperintensity on the hepatobiliary phase is a peculiar genetic subtype of HCC with OATP1B3 overexpression, a less aggressive nature, and mature hepatocyte-like molecular/genetic features.






Similar content being viewed by others
Abbreviations
- AFP:
-
Alpha fetoprotein
- CT:
-
Computed tomography
- FNH:
-
Focal nodular hyperplasia
- FOXM:
-
Forkhead box M
- HCA:
-
Hepatocellular adenoma
- HCC:
-
Hepatocellular carcinoma
- HNF:
-
Hepatocyte nuclear factor
- MRI:
-
Magnetic resonance imaging
- MRP:
-
Multidrug-resistance-associated protein
- OATP:
-
Organic anion transporting polypeptide
References
Hamm B, Staks T, Muhler A et al (1995) Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology 195:785–792
Vogl TJ, Kummel S, Hammerstingl R et al (1996) Liver tumors: comparison of MR imaging with Gd-EOB-DTPA and Gd-DTPA. Radiology 200:59–67
Reimer P, Rummeny EJ, Daldrup HE et al (1997) Enhancement characteristics of liver metastases, hepatocellular carcinomas, and hemangiomas with Gd-EOB-DTPA: preliminary results with dynamic MR imaging. Eur Radiol 7:275–280
Huppertz A, Haraida S, Kraus A et al (2005) Enhancement of focal liver lesions at gadoxetic acid-enhanced MR imaging: correlation with histopathologic findings and spiral CT—initial observations. Radiology 234:468–478
Huppertz A, Balzer T, Blakeborough A et al (2004) Improved detection of focal liver lesions at MR imaging: multicenter comparison of gadoxetic acid-enhanced MR images with intraoperative findings. Radiology 230:266–275
Park G, Kim YK, Kim CS, Yu HC, Hwang SB (2010) Diagnostic efficacy of gadoxetic acid-enhanced MRI in the detection of hepatocellular carcinomas: comparison with gadopentetate dimeglumine. Br J Radiol 83:1010–1016
Besa C, Kakite S, Cooper N, Facciuto M, Taouli B (2015) Comparison of gadoxetic acid and gadopentetate dimeglumine-enhanced MRI for HCC detection: prospective crossover study at 3 T. Acta Radiol Open 4:2047981614561285
Di Martino M, Marin D, Guerrisi A et al (2010) Intraindividual comparison of gadoxetate disodium-enhanced MR imaging and 64-section multidetector CT in the detection of hepatocellular carcinoma in patients with cirrhosis. Radiology 256:806–816
Sano K, Ichikawa T, Motosugi U et al (2011) Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology 261:834–844
Semaan S, Vietti Violi N, Lewis S et al (2020) Hepatocellular carcinoma detection in liver cirrhosis: diagnostic performance of contrast-enhanced CT vs. MRI with extracellular contrast vs. gadoxetic acid. Eur Radiol 30(2):1020–1030. https://doi.org/10.1007/s00330-019-06458-4
The International Consensus Group for Hepatocellular Neoplasia (2009) Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology 49:658–664
Kitao A, Matsui O, Yoneda N et al (2011) The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol 21:2056–2066
Kogita S, Imai Y, Okada M et al (2010) Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol 20:2405–2413
Channual S, Tan N, Siripongsakun S, Lassman C, Lu DS, Raman SS (2015) Gadoxetate disodium-enhanced MRI to differentiate dysplastic nodules and grade of hepatocellular carcinoma: correlation with histopathology. AJR Am J Roentgenol 205:546–553
Narita M, Hatano E, Arizono S et al (2009) Expression of OATP1B3 determines uptake of Gd-EOB-DTPA in hepatocellular carcinoma. J Gastroenterol 44:793–798
Tsuboyama T, Onishi H, Kim T et al (2010) Hepatocellular carcinoma: hepatocyte-selective enhancement at gadoxetic acid-enhanced MR imaging—correlation with expression of sinusoidal and canalicular transporters and bile accumulation. Radiology 255:824–833
Kitao A, Zen Y, Matsui O et al (2010) Hepatocellular carcinoma: signal intensity at gadoxetic acid-enhanced MR imaging—correlation with molecular transporters and histopathologic features. Radiology 256:817–826
Lee SA, Lee CH, Jung WY et al (2011) Paradoxical high signal intensity of hepatocellular carcinoma in the hepatobiliary phase of Gd-EOB-DTPA enhanced MRI: initial experience. Magn Reson Imaging 29:83–90
Ueno A, Masugi Y, Yamazaki K et al (2014) OATP1B3 expression is strongly associated with Wnt/beta-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol 61:1080–1087
Miura T, Ban D, Tanaka S et al (2015) Distinct clinicopathological phenotype of hepatocellular carcinoma with ethoxybenzyl-magnetic resonance imaging hyperintensity: association with gene expression signature. Am J Surg 210:561–569
Kitao A, Matsui O, Yoneda N et al (2012) Hypervascular hepatocellular carcinoma: correlation between biologic features and signal intensity on gadoxetic acid-enhanced MR images. Radiology 265:780–789
Yamashita T, Kitao A, Matsui O et al (2014) Gd-EOB-DTPA-enhanced magnetic resonance imaging and alpha-fetoprotein predict prognosis of early-stage hepatocellular carcinoma. Hepatology 60:1674–1685
Choi JW, Lee JM, Kim SJ et al (2013) Hepatocellular carcinoma: imaging patterns on gadoxetic acid-enhanced MR images and their value as an imaging biomarker. Radiology 267:776–786
European Society of Radilogy (ESR) (2013) ESR statement on the stepwise development of imaging biomarkers. Insights Imaging 4:147–152
Motosugi U, Ichikawa T, Sou H et al (2009) Dilution method of gadolinium ethoxybenzyl diethylenetriaminepentaacetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance imaging (MRI). J Magn Reson Imaging 30:849–854
Motosugi U, Ichikawa T, Sano K et al (2011) Double-dose gadoxetic acid-enhanced magnetic resonance imaging in patients with chronic liver disease. Invest Radiol 46:141–145
Haradome H, Grazioli L, Tsunoo M et al (2010) Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging 32:334–340
Tanimoto A, Higuchi N, Ueno A (2012) Reduction of ringing artifacts in the arterial phase of gadoxetic acid-enhanced dynamic MR imaging. Magn Reson Med Sci 11:91–97
Davenport MS, Viglianti BL, Al-Hawary MM et al (2013) Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology 266:452–461
Davenport MS, Bashir MR, Pietryga JA, Weber JT, Khalatbari S, Hussain HK (2014) Dose-toxicity relationship of gadoxetate disodium and transient severe respiratory motion artifact. AJR Am J Roentgenol 203:796–802
Agrawal MD, Spincemaille P, Mennitt KW et al (2013) Improved hepatic arterial phase MRI with 3-second temporal resolution. J Magn Reson Imaging 37:1129–1136
Kim DH, Choi SH, Byun JH et al (2019) Arterial subtraction images of gadoxetate-enhanced MRI improve diagnosis of early-stage hepatocellular carcinoma. J Hepatol 71:534–542
Song JS, Choi EJ, Park EH, Lee JH (2018) Comparison of transient severe motion in gadoxetate disodium and gadopentetate dimeglumine-enhanced MRI: effect of modified breath-holding method. Eur Radiol 28:1132–1139
Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR (2014) Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology 271:426–434
Bashir MR, Husarik DB, Ziemlewicz TJ, Gupta RT, Boll DT, Merkle EM (2012) Liver MRI in the hepatocyte phase with gadolinium-EOB-DTPA: does increasing the flip angle improve conspicuity and detection rate of hypointense lesions? J Magn Reson Imaging 35:611–616
Haradome H, Grazioli L, Al Manea K et al (2012) Gadoxetic acid disodium-enhanced hepatocyte phase MRI: can increasing the flip angle improve focal liver lesion detection? J Magn Reson Imaging 35:132–139
Gupta RT, Marin D, Boll DT et al (2012) Hepatic hemangiomas: difference in enhancement pattern on 3T MR imaging with gadobenate dimeglumine versus gadoxetate disodium. Eur J Radiol 81:2457–2462
Doo KW, Lee CH, Choi JW, Lee J, Kim KA, Park CM (2009) “Pseudo washout” sign in high-flow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol 193:W490–W496
Tateyama A, Fukukura Y, Takumi K, Shindo T, Kumagae Y, Nakamura F (2016) Hepatic hemangiomas: factors associated with pseudo washout sign on Gd-EOB-DTPA-enhanced MR imaging. Magn Reson Med Sci 15:73–82
Kang Y, Lee JM, Kim SH, Han JK, Choi BI (2012) Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 264:751–760
Lee SM, Lee JM, Ahn SJ, Kang HJ, Yang HK, Yoon JH (2019) LI-RADS version 2017 versus version 2018: diagnosis of hepatocellular carcinoma on gadoxetate disodium-enhanced MRI. Radiology 292:655–663
Nassif A, Jia J, Keiser M et al (2012) Visualization of hepatic uptake transporter function in healthy subjects by using gadoxetic acid-enhanced MR imaging. Radiology 264:741–750
Leonhardt M, Keiser M, Oswald S et al (2010) Hepatic uptake of the magnetic resonance imaging contrast agent Gd-EOB-DTPA: role of human organic anion transporters. Drug Metab Dispos 38:1024–1028
Jia J, Puls D, Oswald S et al (2014) Characterization of the intestinal and hepatic uptake/efflux transport of the magnetic resonance imaging contrast agent gadolinium-ethoxylbenzyl-diethylenetriamine-pentaacetic acid. Invest Radiol 49:78–86
Abe T, Kakyo M, Tokui T et al (1999) Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem 274:17159–17163
Konig J, Cui Y, Nies AT, Keppler D (2000) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168
Ieiri I, Higuchi S, Sugiyama Y (2009) Genetic polymorphisms of uptake (OATP1B1, 1B3) and efflux (MRP2, BCRP) transporters: implications for inter-individual differences in the pharmacokinetics and pharmacodynamics of statins and other clinically relevant drugs. Expert Opin Drug Metab Toxicol 5:703–729
Benness G, Khangure M, Morris I et al (1996) Hepatic kinetics and magnetic resonance imaging of gadolinium-EOB-DTPA in dogs. Invest Radiol 31:211–217
Grazioli L, Bondioni MP, Haradome H et al (2012) Hepatocellular adenoma and focal nodular hyperplasia: value of gadoxetic acid-enhanced MR imaging in differential diagnosis. Radiology 262:520–529
Yoneda N, Matsui O, Kitao A et al (2012) Hepatocyte transporter expression in FNH and FNH-like nodule: correlation with signal intensity on gadoxetic acid enhanced magnetic resonance images. Jpn J Radiol 30:499–508
An HS, Park HS, Kim YJ, Jung SI, Jeon HJ (2013) Focal nodular hyperplasia: characterisation at gadoxetic acid-enhanced MRI and diffusion-weighted MRI. Br J Radiol 86:20130299
Fujiwara H, Sekine S, Onaya H, Shimada K, Mikata R, Arai Y (2011) Ring-like enhancement of focal nodular hyperplasia with hepatobiliary-phase Gd-EOB-DTPA-enhanced magnetic resonance imaging: radiological-pathological correlation. Jpn J Radiol 29:739–743
Kitao A, Matsui O, Yoneda N et al (2018) Differentiation between hepatocellular carcinoma showing hyperintensity on the hepatobiliary phase of gadoxetic acid-enhanced MRI and focal nodular hyperplasia by CT and MRI. AJR Am J Roentgenol 211:347–357
Bioulac-Sage P, Laumonier H, Couchy G et al (2009) Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology 50:481–489
Ba-Ssalamah A, Antunes C, Feier D et al (2015) Morphologic and molecular features of hepatocellular adenoma with gadoxetic acid-enhanced MR imaging. Radiology 277:104–113
Fukusato T, Soejima Y, Kondo F et al (2015) Preserved or enhanced OATP1B3 expression in hepatocellular adenoma subtypes with nuclear accumulation of beta-catenin. Hepatol Res 45:E32–E42
Sekine S, Ogawa R, Ojima H, Kanai Y (2011) Expression of SLCO1B3 is associated with intratumoral cholestasis and CTNNB1 mutations in hepatocellular carcinoma. Cancer Sci 102:1742–1747
Matsui O, Kadoya M, Kameyama T et al (1991) Benign and malignant nodules in cirrhotic livers: distinction based on blood supply. Radiology 178:493–497
Hayashi M, Matsui O, Ueda K et al (1999) Correlation between the blood supply and grade of malignancy of hepatocellular nodules associated with liver cirrhosis: evaluation by CT during intraarterial injection of contrast medium. AJR Am J Roentgenol 172:969–976
Matsui O, Kobayashi S, Sanada J et al (2011) Hepatocellular nodules in liver cirrhosis: hemodynamic evaluation (angiography-assisted CT) with special reference to multi-step hepatocarcinogenesis. Abdom Imaging 36:264–272
Marrero JA, Kulik LM, Sirlin CB et al (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68:723–750
Toyoda H, Kumada T, Tada T et al (2013) Non-hypervascular hypointense nodules detected by Gd-EOB-DTPA-enhanced MRI are a risk factor for recurrence of HCC after hepatectomy. J Hepatol 58:1174–1180
Kim TH, Woo S, Han S, Suh CH, Lee DH, Lee JM (2019) Hepatobiliary phase hypointense nodule without arterial phase hyperenhancement: are they at risk of HCC recurrence after ablation or surgery? A systematic review and meta-analysis. Eur Radiol. https://doi.org/10.1007/s00330-019-06499-9
Kobayashi S, Matsui O, Gabata T et al (2012) Intranodular signal intensity analysis of hypovascular high-risk borderline lesions of HCC that illustrate multi-step hepatocarcinogenesis within the nodule on Gd-EOB-DTPA-enhanced MRI. Eur J Radiol 81:3839–3845
Cannella R, Calandra A, Cabibbo G, Midiri M, Tang A, Brancatelli G (2019) Hyperintense nodule-in-nodule on hepatobiliary phase arising within hypovascular hypointense nodule: outcome and rate of hypervascular transformation. Eur J Radiol 120:108689
Yoneda N, Matsui O, Kitao A et al (2013) Hypervascular hepatocellular carcinomas showing hyperintensity on hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging: a possible subtype with mature hepatocyte nature. Jpn J Radiol 31:480–490
Monga SP (2015) β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology 148:1294–1310
Miyoshi Y, Iwao K, Nagasawa Y et al (1998) Activation of the beta-catenin gene in primary hepatocellular carcinomas by somatic alterations involving exon 3. Cancer Res 58:2524–2527
de La Coste A, Romagnolo B, Billuart P et al (1998) Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A 95:8847–8851
Hoshida Y, Nijman SM, Kobayashi M et al (2009) Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res 69:7385–7392
Kitao A, Matsui O, Yoneda N et al (2015) Hepatocellular carcinoma with beta-catenin mutation: imaging and pathologic characteristics. Radiology 275:708–717
Trauner M, Halilbasic E (2011) Nuclear receptors as new perspective for the management of liver diseases. Gastroenterology 140(1120–1125):e1121–e1112
Wang X, Kiyokawa H, Dennewitz MB, Costa RH (2002) The Forkhead box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A 99:16881–16886
Crestani M, De Fabiani E, Caruso D et al (2004) LXR (liver X receptor) and HNF-4 (hepatocyte nuclear factor-4): key regulators in reverse cholesterol transport. Biochem Soc Trans 32:92–96
Halilbasic E, Claudel T, Trauner M (2013) Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol 58:155–168
Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ (2012) Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem 287:7345–7356
Ning BF, Ding J, Yin C et al (2010) Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res 70:7640–7651
Hatziapostolou M, Polytarchou C, Aggelidou E et al (2011) An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 147:1233–1247
Kitao A, Matsui O, Yoneda N et al (2018) Gadoxetic acid-enhanced magnetic resonance imaging reflects co-activation of beta-catenin and hepatocyte nuclear factor 4alpha in hepatocellular carcinoma. Hepatol Res 48:205–216
Colletti M, Cicchini C, Conigliaro A et al (2009) Convergence of Wnt signaling on the HNF4alpha-driven transcription in controlling liver zonation. Gastroenterology 137:660–672
Segal E, Sirlin CB, Ooi C et al (2007) Decoding global gene expression programs in liver cancer by noninvasive imaging. Nat Biotechnol 25:675–680
Chen J, Wu Z, Xia C et al (2020) Noninvasive prediction of HCC with progenitor phenotype based on gadoxetic acid-enhanced MRI. Eur Radiol 30(2):1232–1242. https://doi.org/10.1007/s00330-019-06414-2
Funding
This study has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Guarantor
The scientific guarantor of this publication is Toshifumi Gabata, M.D., Ph.D.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required because this is a review article.
Ethical approval
Institutional review board approval was not required because this is a review article.
Methodology
• review article
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kitao, A., Matsui, O., Yoneda, N. et al. Gadoxetic acid-enhanced MR imaging for hepatocellular carcinoma: molecular and genetic background. Eur Radiol 30, 3438–3447 (2020). https://doi.org/10.1007/s00330-020-06687-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-06687-y