Abstract
Objectives
The aim of the present study is to analyze preclinical and clinical data on the performance of the currently US Food and Drug Administration (FDA)–approved microwave ablation (MWA) systems.
Methods
A review of the literature, published between January 1, 2005, and December 31, 2016, on seven FDA-approved MWA systems, was conducted. Ratio of ablation zone volume to applied energy R(AZ:E) and sphericity indices were calculated for ex vivo and in vivo experiments.
Results
Thirty-four studies with ex vivo, in vivo, and clinical data were summarized. In total, 14 studies reporting data on ablation zone volume and applied energy were included for comparison R(AZ:E). A significant correlation between volume and energy was found for the ex vivo experiments (r = 0.85, p < 0.001) in contrast to the in vivo experiments (r = 0.54, p = 0.27).
Conclusion
Manufacturers’ algorithms on microwave ablation zone sizes are based on preclinical animal experiments with normal liver parenchyma. Clinical data reporting on ablation zone volume in relation to applied energy and sphericity index during MWA are scarce and require more adequate reporting of MWA data.
Key Points
• Clinical data reporting on the ablation zone volume in relation to applied energy during microwave ablation are scarce.
• Manufacturers’ algorithms on microwave ablation zone sizes are based on preclinical animal experiments with normal liver parenchyma.
• Preclinical data do not predict actual clinical ablation zone volumes in patients with liver tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Thermal ablation such as microwave ablation (MWA) is widely applied for the treatment of liver tumors. Thermal ablation alone or in combination with partial hepatectomy increases the number of intentionally curative treatments in patients in whom partial hepatectomy alone is not an option because of anatomical or functional reasons. Especially in patients with recurrent colorectal liver metastases (CRLM) after previous partial hepatectomy, thermal ablation increases the number of patients who could benefit from repeated procedures [1, 2]. However, the major problem of thermal ablation is incomplete ablation leading to ablation site recurrences (ASR), for which a clear definition should be used [3]. ASR is shown in 60% of patients with a lesion > 5 cm compared to 26% for 3–5 cm lesions and 16% for lesions < 3 cm [4].
Shape and volume of the ablation zone after MWA are depending on physical parameters as thermal conductivity, perfusion rate of the liver parenchyma. These parameters can be different in human liver tissue due to fibrosis, cirrhosis or steatosis [5, 6]. Planning for ablation is partially based on manufacturer-initiated working algorithms in combination with personal experience of the operator. These algorithms, which try to predict the three-dimensional diameter of the ablation zone in relation to the amount of applied energy, are often based on experiments which have serious shortcomings preventing a reliable translation to daily clinical practice. These shortcomings are the result of studies performed in (a) porcine or bovine liver (as opposed to human liver), (b) liver parenchyma (as opposed to tumors), (c) normal liver parenchyma (as opposed to cirrhotic, steatosis, or otherwise non-normal liver parenchyma in humans), and (d) non-perfused ex vivo livers (as opposed to perfused in vivo human livers with variable arterial and portal blood flow). These differences affect the way in which the applied energy is transferred into heat, resulting in highly unpredictable ablation zone volumes [6]. Additionally, despite several individual papers reporting on these shortcomings, a systematic review on this topic is lacking. The aim of this review is to analyze preclinical and clinical data on the performance of the US Food and Drug Administration (FDA)–approved MWA systems. [7]
Methods
Literature search and collected data
A systematic review of seven FDA-approved microwave ablation systems was performed in accordance with the PRISMA statement (Table 1) [13]. Literature published between 1 January 2005 and 1 January 2017, was searched on Scopus including MEDLINE and EMBASE database, using the keywords “microwave ablation” AND “liver.” Retrieved studies were assessed for eligibility based on title and abstract—full papers were obtained and assessed in detail. Studies were included if (a) data on diameter or volume of the ablation zone—based on imaging or pathology—was reported, (b) ablation procedures were performed with FDA-approved MWA systems (Table 1), and (c) the amount of applied energy was reported and (d) were published in English. A data extraction form was used to collect relevant information including type of study (ex vivo, in vivo, or clinical), subject (porcine, bovine, sheep, or human), malignancy (none, primary, or secondary), device, parameters/outcomes, and measurement of ablation zone dimensions (on imaging or gross pathology). Data collection and analysis of unequivocal literature was performed by one researcher. Equivocal papers or data were discussed with co-authors until consensus was obtained. The included studies were categorized in preclinical (animal) and clinical (patient) studies. Preclinical studies were subdivided in two subcategories: ex vivo and in vivo studies (Table 1).
Ablation zone volume and applied energy
Ablation zone volume as reported in the selected papers was recorded. If only long-axis diameter (LAD) and short-axis diameters (SAD) were recorded, the ablation zone volume was estimated, assuming ellipsoid morphology by \( V=\frac{4}{3}\pi \left( LAD/2\right){\left( SAD/2\right)}^2 \). Papers with only one diameter of the ablation zone were excluded for quantitative comparison. Sphericity index of ablation zones with reported SAD and LAD was calculated by SI = SAD2/LAD2.
The cumulative applied energy was determined by multiplying the power level (Watt), as set on the MWA generator, and the ablation time (seconds). The relation between applied energy and ablation zone volume is the only way to quantitatively compare the various published reports. To this end, the ratio of ablation zone volume to applied energy R(AZ:E) of each ablation experiment was calculated by dividing the ablation zone volume (mL) by the applied energy (kJ). The correlation coefficient was determined using IBM SPSS Statistics version 23 (IBM Corporation). The mean volume and applied energy of the subgroups were represented in a bubble chart. The sizes of the bubbles are determined by the sample size of the subgroup.
Results
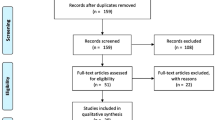
Figure 1 shows a flow diagram of study identification and the exclusion process, resulting in 34 eligible studies [8,9,10,11,12, 14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42] (Tables 2, 3, and 4). Cross-referencing of the identified studies did not reveal any additional papers. In three studies, both ex vivo and in vivo data were published [8,9,10], and these were counted as six separate studies in Table 1. In one study, ex vivo, in vivo, and clinical data were presented [11], and these were counted as three separate studies. Also, in one paper, the ex vivo data of four MWA devices were presented, and these were counted as four separate studies [12]. Assessment of publication bias (overreporting of significant positive results) is not applicable for this review, because the data is not presented as negative or positive. We performed a partial correction for the heterogeneity (variability in the study characteristics) in the analyzed studies by reporting stratified results (for instance ex vivo vs. in vivo and animal vs. human).
Ex vivo animal studies
In total, 18 studies published ex vivo animal results, three in porcine liver [23, 30, 39] and 15 in bovine liver (Table 1) [8,9,10,11,12, 17, 20, 22, 24, 25, 33, 35, 37, 38, 41]. Four studies were performed in perfused liver [25, 30, 37, 38]. Dodd et al used 15 blood-perfused (37 °C) bovine livers for 60 MW ablations with system G (MicroThermX) [37]. Ablation zone volumes, measured by gross pathology, were unaffected by changes in portal venous blood flow (range of 60–100 mL/min per 100 g tissue). These authors also repeated the blood-perfused study with 60 ablations in ten livers with system C (NeuWave) [38]. Again, a change in blood flow rate did not affect the size and shape of the ablation zone, as evaluated by pathology. Pillai et al perfused ex vivo bovine livers with 37 °C Ringer solution and compared volumes and diameters of ablation zones in relation to the distance to the major hepatic vein in three ablation experiments [25]. For system D (AveCure), ablation zones within 8 mm to the major hepatic veins were 22% smaller than ablation zones more than 30 mm away from major hepatic veins [25]. Ringe et al used perfused glass tubes in porcine liver to simulate the hepatic veins [30]. They analyzed 108 ablation zones generated by system E (Evident) and found that ablation zones were influenced by flow rate (0, 700, and 1400 mL/min) at a maximum distance of 10 mm to the glass tube. Hoffmann et al compared the four systems A, B, D, and E (Acculis, Amica, AveCure, and Evident) in bovine liver [12]. They found that system C (NeuWave, 3 antennas) created the largest and most spherical zones. Most systems note in their manual that ablation algorithms are based on “internal ex-vivo experiments.” However, no peer-reviewed publications were found for ex vivo testing for system F (Emprint).
In vivo animal studies
In 12 animal studies, the effects of microwave ablation in in vivo liver parenchyma were analyzed [8,9,10,11, 14, 15, 18, 19, 26,27,28, 34]. Gockner et al compared MWA using system A (Acculis) with and without transarterial embolization before ablation in sheep [19]. Extent and shape of the ablation zones were determined by CT. Ablation zone diameters increased by 22.8% by using transarterial embolization before ablation (p < 0.01). Hines-Peralta et al compared ex vivo bovine and in vivo porcine MWA, using system A (Acculis) [9]. Unexpectedly, 8-mm larger (57 mm vs. 49 mm) diameter of ablation zones (p < 0.01) were obtained in in vivo (57 ± 2 mm) experiments, compared to ex vivo (49 ± 2 mm). However, for ablation times longer than 8 min, ex vivo diameters still increased while in vivo diameters remained constant. Also, Lubner et al compared ex vivo bovine and in vivo porcine MWA [10]. Ablation zones were similar during the first 2 min, but in vivo ablations did not continue to grow as much as ex vivo [10]. No in vivo studies were performed with system D (AveCure) and system F (Emprint).
Clinical studies
Nine studies reported clinical results of the ablation zone [11, 16, 18, 29, 31, 32, 36, 40, 42]. Ratanaprasatporn et al performed a prospective study in ten patients with liver tumors, treated with system D (AveCure) [40]. After liver resection, the ablation zone volumes were measured on gross pathology. Six of the ten treatments showed ablation with complete necrosis on pathological examination [40]. Di Vece et al reported a mean long-axis diameter of 4.85 cm in 20 patients treated with system B (Amica) [42]. Winokur et al analyzed the ablation properties of system B (Amica, 25 ablations in 20 patients) and system C (NeuWave, 11 ablations in 8 patients) in order to analyze if the manufacturer published reference values are useful [16]. The study indicated that in vivo (clinical) ablation zone volumes are significantly smaller than stated by reference values from the manufacturers (0.69 cm3 vs. 1.29 cm3; p = 0.003) [16]. Berber et al compared predicted ablation diameters (system F, Emprint) with the ablation zone at the 2-week post-ablation CT scan of nine patients [29]. The maximum diameter of the ablation zone was 1.12 ± 0.11 times larger than predicted. No residual tumors were seen at the 2-week scan. In another study, Berber reported a scatterplot showing the CT diameter of ablation zones obtained after 100 W of power during laparoscopic MW ablation with system F (Emprint) in 15 patients [31]. In a study of 149 laparoscopic ablations, Zaidi et al reported system F to be satisfactory in achieving the predicted ablation sizes [32]. Amabile et al compared ablation zone dimensions in liver tumors in patients with both in vivo (porcine) and ex vivo (bovine) experiments (only liver parenchyma). They concluded that the ex vivo animal data reliably predicted the dimensions in (in vivo) human liver tumors. Shyn et al compared ablation zone diameters and volumes with applied and net energy (after correcting for reflectivity), and with manufacturer chart predictions [36]. Applied energy (r = 0.52) and net energy (r = 0.53) did not correlate better than manufacturer chart prediction (r = 0.60). Also, no differences were seen between cirrhotic and non-cirrhotic livers. None of the clinical studies were performed with system A (Acculis), E (Evident), and G (MicroThermX).
Comparison of ablation volume–applied energy ratio
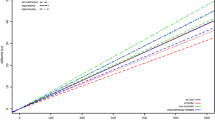
In total, 14 animal studies reported data on ablation zone volume and applied energy and could be used to compare R(AZ:E) [8, 9, 11, 12, 14, 17, 19, 22,23,24, 26, 30, 35, 39]. These studies were categorized in 22 subgroups based on device and tissue (ex vivo and in vivo). A significant correlation between volume and energy was found for the ex vivo experiments (r = 0.85, p < 0.001) in contrast to the in vivo experiments (r = 0.54, p = 0.27) (Fig. 2). Results of SI calculation are shown in Tables 2, 3, and 4.
Discussion
In this review on studies performed with seven FDA-approved MWA devices, we found that only a minority (9 out of 43 studies) was based on clinical studies. On top of that, a major limitation in these clinical studies is the lack of data on the ratio ablation zone volume: applied energy R(AZ:E). In only 14 preclinical studies, the R(AZ:E) was calculated. To our knowledge, this is the first study which compares the ratio ablation zone volume and applied energy of FDA-approved MWA devices between 2005 and 2017. MWA systems are categorized as class II medical devices by the FDA. This classification requires that a new device must be proven to be substantially equivalent to a device that was legally marketed (predicate device) prior to May 28, 1976. Since microwave technology has been around for decades, ex vivo bench testing is sufficient for the regulatory approvals and no additional submission of clinical data is required [7]. Also, reporting results on ablation zone volume and applied energy in clinical setting are limited. This might be the reason for the lack of clinical data of MWA systems.
An analysis of the studies in our review suggests a number of possible explanations for the discrepancy between manufacturers’ provided ablation algorithms and the actual clinical ablation zone sizes. This discrepancy was demonstrated by Winokur et al who indicated that in vivo (clinical) ablation zone volumes are significantly smaller than stated by reference values from the manufacturers of the Amica and NeuWave system [16]. Depending on the type of study (ex vivo vs. in vivo), ablation time was found to be a determinant for ablation zone diameter. For ablation time longer than 8 min, ex vivo diameter still increased while in vivo diameters remained constant, suggesting plateau formation which probably is caused by the antagonizing effect of perfusion [9, 16].
Obviously, preclinical studies using animal livers are only performed in normal liver parenchyma with absence of tumor tissue (tumor characteristics) and underlying liver disease (liver characteristics). Deshazer et al simulated these properties in a two-compartmental computer model and showed that ablation zone volume could increase with 36% in patients with cirrhotic liver as compared to healthy liver tissue [6]. Tumor tissue revealed a 20% higher thermal conductivity (the property of tissue to conduct heat) than healthy liver tissue. Hyperperfused and hypoperfused tumors within normal liver parenchyma showed minimal variation in ablation zone volume. Furthermore, steatotic parenchyma had 50% lower thermal conductivity than healthy liver tissue [6]. Hepatic steatosis is of importance in patients with CRLM treated with neo-adjuvant chemotherapy and should be taken into account when planning MWA treatment for these patients [43]. Perfusion in ex vivo experiments also determines to a great extent the effect of heat distribution by convection in MWA; convection of heat is mainly influenced by vascularization of the tumor and adjacent large blood vessels which can cause heat sink. In most ex vivo studies, livers were not perfused and thus not subject to heat sink effects. Perfusion of cirrhotic liver tissue is reported to be 36% lower than healthy liver tissue [44]. This is of importance because approximately 90% of the patients with HCC suffer from cirrhosis [45]. Additionally, the majority of HCCs have a predominant arterial perfusion, which makes prediction of the obtained ablation zone in relation to perfusion phenomena even more imprecise. Compared to perfusion in liver parenchyma, HCC tumor tissue has a significantly higher arterial perfusion and lower portal venous hepatic blood flow [46]. Therefore, the results of Amabile et al are difficult to interpret, because they found a better correlation between ablation zone dimensions in liver tumors (HCC) in patients and non-perfused (ex vivo) bovine liver than in perfused (in vivo) porcine liver. A possible explanation for this seemingly contradictory finding is that less than 20% of the ablation zone volume encompasses tumor and more than 80% liver parenchyma [47]. This suggests that liver parenchyma might be of more importance for the ablation zone volume than the tumor tissue. Clinical studies were limited in number and additionally in general no distinction in tumor type (primary or secondary) is made. None of the studies in this review reported the underlying liver diseases, like hepatic steatosis, fibrosis, or cirrhosis. In a retrospective study, MWA volumes of HCC in cirrhotic liver and CRLM in healthy liver were compared. R(AZ:E) for HCC with system A (Acculis) is twofold higher for HCC (R(AZ:E) = 0.61) compared to CRLM (R(AZ:E) = 0.35). Also, ablation treatment for HCC with system B (Amica) resulted in a 50% increase of ablation zone volume, compared to CRLM [47]. For treatment of HCC up to 4 cm, no significant differences were found in local tumor progression between radiofrequency ablation (RFA) and MWA [48].
During liver ablation, the power of heating (Watt) is an important factor. When using low power, relatively more heat will be dispersed into the surrounding tissue, and temperature of the ablation zone will be low. In contrast, higher power for short time will lead to high temperatures around the antenna and contraction of the target tissue. Bedoya et al compared different ways to deliver 30 kJ of energy (25 W 20 min, 50 W 10 min, and 100 W 5 min) in in vivo porcine livers [27]. Significantly larger ablation zone volumes were achieved with high power ablations (23.6 ± 26.5 mL; 105.4 ± 78.3 mL; 265.7 ± 208.1, respectively; p < 0.03). Interestingly, they also investigated the effect of pulsed energy delivery (25 kJ) to limit the effects of heat sink and to provide larger ablation zone volumes than continuous energy delivery (67.4 ± 34.5 cm3 vs. 23.6 ± 26.5 cm3, p = 0.43).
Another important factor is the reflection of energy by the antenna cable. The majority of the microwave systems report the output power of the generator as the applied energy at the antenna. However, 15–30% of the output energy per meter length will be lost by the antenna cable [49]. So, for comparison of ablation devices by R(AZ:E), the most relevant parameter is the amount of energy deposited into the liver tissue, which is known as the net energy [5, 49, 50]. Only systems B (Amica) and D (AveCure) display the reflection of energy on the generator. A comparable disagreement is found for the various antenna designs: if an antenna is built to have stronger fields in certain areas (Watts/area), a different amount of tissue reaching 60 °C might be expected. So, differences in R(AZ:E) between devices might be due to antenna design and cable length [49]. Despite the limitation of using R(AZ:E), it is the only parameter to compare the currently available data quantitatively.
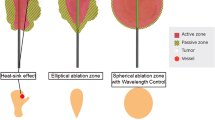
Most liver tumors treated by MWA are spherical which requires also a spherical ablation zone to achieve a sufficient ablation margin. However, most ablation systems create ellipsoidal ablation zones with poor sphericity values. Reflection of energy by the antenna shaft results in heating of the shaft which contributes to bigger LAD. This may also increase the risk of thermal damage of adjacent liver tissue. Data about the sphericity of ablation zones in patients are scarce. In this study, we calculated the SI for all studies in which the LAD and SAD were reported. However, data are heterogeneous and depending on power and time of the ablation. A recently published study compared system F (Emprint) with systems B (Amica) and E (Evident) [51]. Significantly more spherical ablation zones in patients were achieved with system F than with systems B and E. Complete ablation was possible with a single antenna placement regardless of the angle approach because of the almost spherical ablation zone.
There might be several strategies to decrease ASR after microwave ablation. First of all, more in vivo and clinical studies with MWA should be conducted with cirrhotic and other diseased parenchyma, like steatotic liver, to confirm computer model studies. Secondly, presentation of data in studies should be reported adequately by means of applied energy (without reflection), LAD, SAD, SI, and volume of each ablation zone (Table 5). Additionally, for clinical studies, the status of the underlying liver parenchyma should be reported.
There are some limitations to this study. Ablation outcomes were compared by R(AZ:E). Ablation zone volume as reported in the selected papers was recorded. If only one or two diameters were reported, the ablation zone volume was estimated assuming ellipsoid or spherical morphology. Also, the diameters or volumes of the ablation zones were assessed by different measurement modalities (gross pathology, CT, MRI, or ultrasound) which induce bias. Finally, a systematic review formally requires a control group, but it is clear that this is not available for the current review.
In conclusion, manufacturers’ algorithms on microwave ablation zone sizes are based on preclinical animal experiments with normal liver parenchyma. Clinical data reporting on ablation zone volume in relation to applied energy and sphericity index during MWA are scarce which requires more adequate reporting on MWA data.
Abbreviations
- ASR:
-
Ablation site recurrences
- CRLM:
-
Colorectal liver metastases
- FDA:
-
US Food and Drug Administration
- HCC:
-
Hepatocellular carcinoma
- LAD:
-
Long-axis diameter
- MWA:
-
Microwave ablation
- R(AZ:E):
-
Ratio of ablation zone volume to applied energy
- SAD:
-
Short-axis diameters
- SI:
-
Sphericity index
References
Hof J, Wertenbroek MW, Peeters PM, Widder J, Sieders E, de Jong KP (2016) Outcomes after resection and/or radiofrequency ablation for recurrence after treatment of colorectal liver metastases. Br J Surg 103:1055–1062
Meijerink MR, Puijk RS, van Tilborg AAJM et al (2018) Radiofrequency and microwave ablation compared to systemic chemotherapy and to partial hepatectomy in the treatment of colorectal liver metastases: a systematic review and metaanalysis: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. https://doi.org/10.1007/s00270-018-1959-3
Goldberg SN, Grassi CJ, Cardella JF et al (2009) Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol 20:S377–S390
Mulier S, Ni Y, Jamart J, Ruers T, Marchal G, Michel L (2005) Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–171
Brace CL (2009) Microwave ablation technology: what every user should know. Curr Probl Diagn Radiol 38:61–67
Deshazer G, Merck D, Hagmann M, Dupuy DE, Prakash P (2016) Physical modeling of microwave ablation zone clinical margin variance. Med Phys 43:1764. https://doi.org/10.1118/1.4942980
Ward RC, Healey TT, Dupuy DE (2013) Microwave ablation devices for interventional oncology. Expert Rev Med Devices 10:225–238. https://doi.org/10.1586/erd.12.77
Harari CM, Magagna M, Bedoya M et al (2016) Microwave ablation: comparison of simultaneous and sequential activation of multiple antennas in liver model systems. Radiology 278:95–103
Hines-Peralta AU, Pirani N, Clegg P et al (2006) Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology 239:94–102
Lubner MG, Hinshaw JL, Andreano A, Sampson L, Lee FT Jr, Brace CL (2012) High-powered microwave ablation with a small-gauge, gas-cooled antenna: initial ex vivo and in vivo results. J Vasc Interv Radiol 23:405–411
Amabile C, Ahmed M, Solbiati L et al (2017) Microwave ablation of primary and secondary liver tumours : ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia 33:34–42
Hoffmann R, Rempp H, Erhard L et al (2013) Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology 268:89–97
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.. Ann Intern Med 151:264–269
Awad MM, Devgan L, Kamel IR, Torbensen M, Choti MA (2007) Microwave ablation in a hepatic porcine model: correlation of CT and histopathologic findings. HPB (Oxford) 9:357–362
Garrean S, Hering J, Saied A et al (2009) Ultrasound monitoring of a novel microwave ablation (MWA) device in porcine liver: lessons learned and phenomena observed on ablative effects near major intrahepatic vessels. J Gastrointest Surg 13:334–340
Winokur RS, Du JY, Pua BB et al (2014) Characterization of in vivo ablation zones following percutaneous microwave ablation of the liver with two commercially available devices: are manufacturer published reference values useful? J Vasc Interv Radiol 25:1939–1946.e1
Liu D, Brace CL (2014) CT imaging during microwave ablation: analysis of spatial and temporal tissue contraction. Med Phys 41:113303
Correa-Gallego C, Karkar AM, Monette S, Ezell PC, Jarnagin WR, Kingham TP (2014) Intraoperative ultrasound and tissue elastography measurements do not predict the size of hepatic microwave ablations. Acad Radiol 21:72–78
Gockner TL, Zelzer S, Mokry T et al (2015) Sphere-enhanced microwave ablation (sMWA) versus bland microwave ablation (bMWA): technical parameters, specific CT 3D rendering and histopathology. Cardiovasc Intervent Radiol 38:442–452
Niemeyer DJ, Simo KA, McMillan MT et al (2015) Optimal ablation volumes are achieved at submaximal power settings in a 2.45-GHz microwave ablation system. Surg Innov 22:41–45
Cavagnaro M, Pinto R, Lopresto V (2015) Numerical models to evaluate the temperature increase induced by ex vivo microwave thermal ablation. Phys Med Biol 60:3287–3311. https://doi.org/10.1088/0031-9155/60/8/3287
Cavagnaro M, Amabile C, Cassarino S, Tosoratti N, Pinto R, Lopresto V (2015) Influence of the target tissue size on the shape of ex vivo microwave ablation zones. Int J Hyperthermia 31:48–57
Paul J, Vogl TJ, Chacko A (2015) Dual energy computed tomography thermometry during hepatic microwave ablation in an ex-vivo porcine model. Phys Med 31:683–691
Kim HJ, Rhim H, Lee MW, Jeong WK (2015) Measurement of intrahepatic pressure during microwave ablation in an ex vivo bovine liver model. Gut Liver 9:784
Pillai K, Akhter J, Chua TC et al (2015) Heat sink effect on tumor ablation characteristics as observed in monopolar radiofrequency, bipolar radiofrequency, and microwave, using ex vivo calf liver model. Medicine (Baltimore) 94:e580
Meloni MF, Andreano A, Bovo G et al (2011) Acute portal venous injury after microwave ablation in an in vivo porcine model: a rare possible complication. J Vasc Interv Radiol 22:947–951
Bedoya M, del Rio AM, Chiang J, Brace CL (2014) Microwave ablation energy delivery: Influence of power pulsing on ablation results in an ex vivo and in vivo liver model. Med Phys 41:123301
Moreland AJ, Lubner MG, Ziemlewicz TJ et al (2015) Evaluation of a Thermoprotective gel for Hydrodissection during percutaneous microwave ablation: in vivo results. Cardiovasc Intervent Radiol 38:722–730
Berber E (2015) The first clinical application of planning software for laparoscopic microwave thermosphere ablation of malignant liver tumours. HPB (Oxford) 17:632–636
Ringe KI, Lutat C, Rieder C, Schenk A, Wacker F, Raatschen HJ (2015) Experimental evaluation of the heat sink effect in hepatic microwave ablation. PLoS One 10(7):e0134301
Berber E (2016) Laparoscopic microwave thermosphere ablation of malignant liver tumors: an initial clinical evaluation. Surg Endosc 30:692–698
Zaidi N, Okoh A, Yigitbas H, Yazici P, Ali N, Berber E (2016) Laparoscopic microwave thermosphere ablation of malignant liver tumors: an analysis of 53 cases. J Surg Oncol 113:130–134
Weiss N, Goldberg SN, Nissenbaum Y, Sosna J, Azhari H (2015) Planar strain analysis of liver undergoing microwave thermal ablation using x-ray CT. Med Phys 42:372–380
Wu PH, Brace CL (2016) Analysis of iodinated contrast delivered during thermal ablation: is material trapped in the ablation zone? Phys Med Biol 61:6041–6054
Lopresto V, Pinto R, Lovisolo GA, Cavagnaro M (2012) Changes in the dielectric properties of ex vivo bovine liver during microwave thermal ablation at 2.45 GHz. Phys Med Biol 57:2309–2327
Shyn PB, Bird JR, Koch RM et al (2016) Hepatic microwave ablation zone size: correlation with Total energy, net energy, and manufacturer-provided chart predictions. J Vasc Interv Radiol 27:1389–1396
Dodd GD 3rd, Dodd NA, Lanctot AC, Glueck DA (2013) Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 267:129–136
Dodd GD 3rd, Kreidler SM, Lanctot AC, Glueck DH (2015) Effect of change in portal venous blood flow rates on the performance of a 2.45-GHz microwave ablation device. Radiology 277:727–732
Sommer CM, Bryant M, Kortes N et al (2012) Microwave ablation in porcine livers applying 5-minute protocols: influence of deployed energy on extent and shape of coagulation. J Vasc Interv Radiol 23:1692–1699
Ratanaprasatporn L, Charpentier KP, Resnick M, Lu S, Dupuy D (2013) Intra-operative microwave ablation of liver malignancies with tumour permittivity feedback control: a prospective ablate and resect study. HPB (Oxford) 15:997–1001
Collettini F, Rathke H, Schnackenburg B et al (2013) Fluid preinjection for microwave ablation in an ex vivo bovine liver model assessed with volumetry in an open MRI system. Diagn Interv Radiol 19:427–432
Di Vece F, Tombesi P, Ermili F, Maraldi C, Sartori S (2014) Coagulation areas produced by cool-tip radiofrequency ablation and microwave ablation using a device to decrease back-heating effects: a prospective pilot study. Cardiovasc Intervent Radiol 37:723–729. https://doi.org/10.1007/s00270-013-0733-9
Robinson SM, Wilson CH, Burt AD, Manas DM, White SA (2012) Chemotherapy-associated liver injury in patients with colorectal liver metastases: a systematic review and Meta-analysis. Ann Surg Oncol 19:4287–4299
Van Beers BE, Leconte I, Materne R, Smith AM, Jamart J, Horsmans Y (2001) Hepatic perfusion parameters in chronic liver disease. AJR Am J Roentgenol 176:667–673
Ioannou GN, Splan MF, Weiss NS, McDonald GB, Beretta L, Lee SP (2007) Incidence and predictors of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 5:938–945
Taouli B, Johnson RS, Hajdu CH et al (2013) Hepatocellular carcinoma: perfusion quantification with dynamic contrast-enhanced MRI. AJR Am J Roentgenol 201:795–800
Heerink WJ, Solouki AM, Vliegenthart R et al (2018) The relationship between applied energy and ablation zone volume in patients with hepatocellular carcinoma and colorectal liver metastasis. Eur Radiol. https://doi.org/10.1007/s00330-017-5266-1
Vietti Violi N, Duran R, Guiu B et al (2018) Efficacy of microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol 3:317–325
Poggi G, Tosoratti N, Montagna B, Picchi C (2015) Microwave ablation of hepatocellular carcinoma. World J Hepatol 7:2578–2589
Ahmed M, Solbiati L, Brace CL et al (2014) Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology 273:241–260
Vogl TJ, Basten LM, Nour-Eldin NA et al (2018) Evaluation of microwave ablation of liver malignancy with enabled constant spatial energy control to achieve a predictable spherical ablation zone. Int J Hyperthermia 34:492–500
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. K.P. de Jong.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because this is a systematic review.
Ethical approval
Institutional Review Board approval was not required because this is a systematic review.
Methodology
• Systematic review
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ruiter, S.J.S., Heerink, W.J. & de Jong, K.P. Liver microwave ablation: a systematic review of various FDA-approved systems. Eur Radiol 29, 4026–4035 (2019). https://doi.org/10.1007/s00330-018-5842-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5842-z