Abstract
Purpose
MRI has limited ability to detect multifocal disease or the full extent of prostate involvement with clinically significant prostate cancer (sPC). We compare the spatial co-localization at sextant resolution of MRI lesions and histopathological mapping by combined targeted and extended systematic biopsies.
Materials and methods
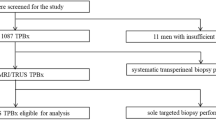
Sextants were mapped for sPC (ISUP group ≥ 2) by 24-core transperineal systematic biopsies in 316 patients with suspicion for sPC and by MR lesions of PI-RADS score of ≥ 3. The gold standard is combined systematic (median 23 cores) and targeted biopsies.
Results
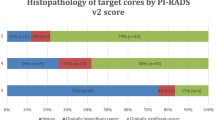
Of 316 men, 121 (38%) harbored sPC. Of these 121 patients, 4 (3%) had a negative MRI. MRI correctly identified 117/121 (97%) patients with sPC. In these patients, mpMRI missed no additional sPC in 96 (82%), while MRI-negative sPC lesions were present in 21 patients (18%). Of 1896 sextants, 379 (20%) harbored sPC. MR-positive sextants contained sPC in 26% (337/1275), compared to 7% (42/621) in MR-negative sextants. On a patient basis, sensitivity was 0.97, specificity 0.22, positive predictive value 0.43, and negative predictive value 0.91. On a sextant basis, sensitivity was 0.73, specificity 0.38, positive predictive value 0.26, and negative predictive value 0.93.
Conclusion
MpMRI mapping agreed well with histopathology with, at the observed sPC prevalence and on a patient basis, excellent sensitivity and negative predictive value, and acceptable specificity and positive predictive value for sPC. However, 18% of sPC was outside the mpMRI mapped region, quantifying limitations of MRI for complete localization of disease extent.
Key Points
• Currently, exclusive MRI mapping of the prostate for focal treatment planning cannot be recommended, as significant prostate cancer may remain untreated in a substantial number of cases.
• At the observed sPC prevalence and on a patient basis, mpMRI has excellent sensitivity and NPV, and acceptable specificity and PPV for detection of prostate cancer, supporting its use to detect suspicious lesions before biopsy.
• Despite the excellent global performance, 18% of sPC was outside the mpMRI mapped region even when a security margin of 10 mm was considered, indicating that prostate MRI has limited ability to completely map all cancer foci within the prostate.




Similar content being viewed by others
Abbreviations
- AS:
-
Active surveillance
- Bx:
-
Biopsy
- DCE:
-
Dynamic contrast-enhanced imaging
- DRE:
-
Digital-rectal examination
- DWI:
-
Diffusion-weighted imaging
- EPI:
-
Echo-planar imaging
- GP:
-
Gleason pattern
- GS:
-
Gleason score
- ISUP:
-
International Society of Urological Pathology
- mpMRI:
-
Multiparametric magnetic resonance imaging
- MRI:
-
Magnetic resonance imaging
- NPV:
-
Negative predictive value
- PC:
-
Prostate cancer
- PI-RADS:
-
Prostate Imaging Reporting and Data System
- PPV:
-
Positive predictive value
- PSA:
-
Prostate specific antigen
- RP:
-
Radical prostatectomy
- SB:
-
Systematic transperineal saturation
- sPC:
-
Significant prostate cancer
- STARD:
-
Standards of Reporting of Diagnostic Accuracy
- START:
-
Standards of Reporting for MRI-targeted Biopsy Studies
- TB:
-
MRI-targeted biopsy
- TRUS:
-
Transrectal ultrasound
References
Panebianco V, Barchetti G, Simone G et al (2018) Negative multiparametric magnetic resonance imaging for prostate cancer : what’s next ? Eur Urol. https://doi.org/10.1016/j.eururo.2018.03.007
Barentsz JO, Richenberg J, Clements R et al (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22:746–757. https://doi.org/10.1007/s00330-011-2377-y
Siddiqui MM, Rais-Bahrami S, Truong H et al (2013) Magnetic resonance imaging/ultrasound-fusion biopsy significantly upgrades prostate cancer versus systematic 12-core transrectal ultrasound biopsy. Eur Urol 64:713–719. https://doi.org/10.1016/j.eururo.2013.05.059
Radtke JP, Kuru TH, Boxler S et al (2015) Comparative analysis of transperineal template saturation prostate biopsy versus magnetic resonance imaging targeted biopsy with magnetic resonance imaging-ultrasound fusion guidance. J Urol. https://doi.org/10.1016/j.juro.2014.07.098
Mottet N, Bellmunt J, Bolla M et al (2017) EAU-ESTRO-SIOG guidelines on prostate Cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 71:618–629. https://doi.org/10.1016/j.eururo.2016.08.003
Kasivisvanathan V, Dufour R, Moore CM et al (2013) Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol 189:860–866. https://doi.org/10.1016/j.juro.2012.10.009
Ahmed HU, El-Shater Bosaily A, Brown LC et al (2017) Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet 6736:32401–32401. https://doi.org/10.1016/S0140-6736(16)32401-1
Kasivisvanathan V, Rannikko AS, Borghi M et al (2018) MRI-targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med. https://doi.org/10.1056/NEJMoa1801993
Kenigsberg AP, Llukani E, Deng FM, Melamed J, Zhou M, Lepor H (2018) The use of magnetic resonance imaging to predict oncological control among candidates for focal ablation of prostate cancer. Urology 112:121–125. https://doi.org/10.1016/j.urology.2017.10.014
Moldovan PC, Van den Broeck T, Sylvester R et al (2017) What is the negative predictive value of multiparametric magnetic resonance imaging in excluding prostate cancer at biopsy ? A systematic review and meta-analysis from the european association of urology prostate cancer guidelines panel. Eur Urol. https://doi.org/10.1016/j.eururo.2017.02.026
Borofsky S, George AK, Gaur S et al (2018) What are we missing ? False-negative cancers at multiparametric MR imaging of the prostate 1. Radiology 0:1–10. https://doi.org/10.1148/radiol.2017152877
Radtke JP, Schwab C, Wolf MB et al (2016) Multiparametric magnetic resonance imaging (MRI) and MRI – transrectal ultrasound fusion biopsy for index tumor detection : correlation with radical prostatectomy specimen. Eur Urol 70:846–853. https://doi.org/10.1016/j.eururo.2015.12.052
Bonekamp D, Kohl S, Wiesenfarth M et al (2018) Radiomic machine learning for characterization of prostate lesions by MRI: comparison to ADC values. Radiology. https://doi.org/10.1148/radiol.2018173064
Moore CM, Kasivisvanathan V, Eggener S et al (2013) Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol 64:544–552. https://doi.org/10.1016/j.eururo.2013.03.030
Weinreb JC, Barentsz JO, Choyke PL et al (2015) PI-RADS prostate imaging - reporting and data system: 2015, version 2. Eur Urol 69(1):16–40. https://doi.org/10.1016/j.eururo.2015.08.052
Kuru TH, Wadhwa K, Chang RT et al (2013) Definitions of terms, processes and a minimum dataset for transperineal prostate biopsies: a standardization approach of the Ginsburg Study Group for Enhanced Prostate Diagnostics. BJU Int 112:568–577. https://doi.org/10.1111/bju.12132
Nolden M, Zelzer S, Seitel A et al (2013) The medical imaging interaction toolkit: challenges and advances: 10 years of open-source development. Int J Comput Assist Radiol Surg 8:607–620. https://doi.org/10.1007/s11548-013-0840-8
Fritzsche KH, Neher PF, Reicht I et al (2012) MITK diffusion imaging. Methods Inf Med 51:441–448. https://doi.org/10.3414/ME11-02-0031
Le Nobin J, Rosenkrantz AB, Villers A et al (2015) Image guided focal therapy of MRI-visible prostate cancer: defining a 3D treatment margin based on MRI-histology co-registration analysis. J Urol 1–7. https://doi.org/10.1016/j.juro.2015.02.080
R Development Core Team R (2015) R: a language and environment for statistical computing. Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/. Accessed 12 Aug 2018
Bossuyt PM, Reitsma JB, Bruns DE et al (2003) Towards complete and, accurate reporting of studies of diagnostic accuracy: the STARD initiative. Radiology 226:24–28. https://doi.org/10.1136/bmj.326.7379.41
Baco E, Ukimura O, Rud E et al (2015) Magnetic resonance imaging – transectal ultrasound image-fusion biopsies accurately characterize the index tumor: correlation with step-sectioned radical prostatectomy specimens in 135 patients. Eur Urol 67:787–794. https://doi.org/10.1016/j.eururo.2014.08.077
Mortezavi A, Märzendorfer O, Donati OF et al (2018) Diagnostic accuracy of mpMRI and fusion-guided targeted biopsy evaluated by transperineal template saturation prostate biopsy for the detection and characterization of prostate cancer. J Urol. https://doi.org/10.1016/j.juro.2018.02.067
De Visschere PJ, Naesens L, Libbrecht L et al (2016) What kind of prostate cancers do we miss on multiparametric magnetic resonance imaging? Eur Radiol 26:1098–1107. https://doi.org/10.1007/s00330-015-3894-x
Ullrich T, Quentin M, Arsov C et al (2017) Risk stratification of “equivocal” PI-RADS lesions in mp-MRI of the prostate. J Urol. https://doi.org/10.1016/j.juro.2017.09.074
Meng X, Rosenkrantz AB, Mendhiratta N et al (2016) Relationship of pre-biopsy multiparametric MRI and biopsy indication with MRI-US fusion-targeted prostate biopsy outcomes. Eur Urol 69:512–517. https://doi.org/10.1016/j.eururo.2015.06.005
Venderink W, Van Luijtelaar A, Bomers JG et al (2017) Results of targeted biopsy in men with magnetic resonance imaging lesions classified equivocal , likely or highly Likely to be clinically significant prostate cancer. Eur Urol 1–8. https://doi.org/10.1016/j.eururo.2017.02.021
Le Nobin J, Orczyk C, Deng FM et al (2014) Prostate tumour volumes: evaluation of the agreement between magnetic resonance imaging and histology using novel co-registration software. BJU Int 114:E105–E112. https://doi.org/10.1111/bju.12750
Bratan F, Melodelima C, Souchon R et al (2015) How accurate is multiparametric MR imaging in evaluation of prostate cancer volume? Radiology 275:144–154. https://doi.org/10.1148/radiol.14140524
Sonn GA, Fan RE, Ghanouni P et al (2017) Prostate magnetic resonance imaging interpretation varies substantially across radiologists. Eur Urol Focus. https://doi.org/10.1016/j.euf.2017.11.010
Turkbey B, Pinto PA, Mani H et al (2010) Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology 255:89–99. https://doi.org/10.1148/radiol.09090475
Rosenkrantz AB, Deng FM, Kim S et al (2012) Prostate cancer: multiparametric mri for index lesion localization - a multiple-reader study. AJR Am J Roentgenol 199:830–837. https://doi.org/10.2214/AJR.11.8446
Dickinson L, Ahmed HU, Allen C et al (2011) Magnetic resonance imaging for the detection, localisation, and characterisation of prostate cancer: recommendations from a European consensus meeting. Eur Urol 59:477–494. https://doi.org/10.1016/j.eururo.2010.12.009
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Heinz-Peter Schlemmer.
Conflict of interest
David Bonekamp is a speaker for Profound Medical Inc.
Patrick Schelb has nothing to declare.
Manuel Wiesenfarth has nothing to declare.
Tristan Anselm Kuder has nothing to declare.
Fenja Deister has nothing to declare.
Albrecht Stenzinger declares the following: consulting fee and payment for lectures: Astra Zeneca, BMS, Novartis, Roche, Illumina, Thermo Fisher; travel support: Astra Zeneca, BMS, Novartis, Illumina, Thermo Fisher; board member: Astra Zeneca, BMS, Novartis, Thermo Fisher.
Joanne Nyarangi-Dix has nothing to declare.
Matthias Röthke declares consulting fee and payment for lectures: Siemens Healthineers, Curagita AG.
Markus Hohenfellner has nothing to declare.
Heinz-Peter Schlemmer declares the following: consulting fee or honorarium: Siemens, Curagita, Profound, Bayer; travel support: Siemens, Curagita, Profound, Bayer; board member: Curagita; consultancy: Curagita, Bayer; grants/grants pending: BMBF, Deutsche Krebshilfe, Dietmar-Hopp-Stiftung, Roland-Ernst-Stiftung; payment for lectures: Siemens, Curagita, Profound, Bayer.
Jan Philipp Radtke declares payment for consultant work from Saegeling Medizintechnik and Siemens Heathineers and for development of educational presentations from Saegeling Medizintechnik.
Statistics and biometry
Manuel Wiesenfarth is the lead statistician and co-author on this paper.
Informed consent
Written informed consent was waived by the Ethics Commission.
Ethical approval
Ethical approval was obtained.
Study subjects or cohorts overlap
The examined cohort was subject to a recently published study (Bonekamp D, Kohl S, Wiesenfarth M et al (2018) Radiomic machine learning for characterization of prostate lesions by MRI: comparison to ADC values. Radiology 31:173064. https://doi.org/10.1148/radiol.2018173064. Focusing on apparent diffusion coefficient and radiomics for lesion classification; however, sextant-level histopathology to mpMRI mapping has not been previously performed.
Methodology
• retrospective
• diagnostic study
• single-center study
Rights and permissions
About this article
Cite this article
Bonekamp, D., Schelb, P., Wiesenfarth, M. et al. Histopathological to multiparametric MRI spatial mapping of extended systematic sextant and MR/TRUS-fusion-targeted biopsy of the prostate. Eur Radiol 29, 1820–1830 (2019). https://doi.org/10.1007/s00330-018-5751-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5751-1