Abstract
Objectives
Inherited myopathies are major causes of muscle atrophy and are often characterized by rigid spine syndrome, a clinical feature designating patients with early spinal contractures. We aim to present a decision algorithm based on muscular whole body magnetic resonance imaging (mWB-MRI) as a unique tool to orientate the diagnosis of each inherited myopathy long before the genetically confirmed diagnosis.
Methods
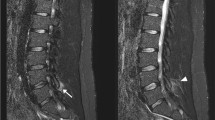
This multicentre retrospective study enrolled 79 patients from referral centres in France, Brazil and Chile. The patients underwent 1.5-T or 3-T mWB-MRI. The protocol comprised STIR and T1 sequences in axial and coronal planes, from head to toe. All images were analyzed manually by multiple raters. Fatty muscle replacement was evaluated on mWB-MRI using both the Mercuri scale and statistical comparison based on the percentage of affected muscle.
Results
Between February 2005 and December 2015, 76 patients with genetically confirmed inherited myopathy were included. They were affected by Pompe disease or harbored mutations in RYR1, Collagen VI, LMNA, SEPN1, LAMA2 and MYH7 genes. Each myopathy had a specific pattern of affected muscles recognizable on mWB-MRI. This allowed us to create a novel decision algorithm for patients with rigid spine syndrome by segregating these signs. This algorithm was validated by five external evaluators on a cohort of seven patients with a diagnostic accuracy of 94.3% compared with the genetic diagnosis.
Conclusion
We provide a novel decision algorithm based on muscle fat replacement graded on mWB-MRI that allows diagnosis and differentiation of inherited myopathies presenting with spinal rigidity.
Key Points
• Inherited myopathies are rare, diagnosis is challenging and genetic tests require specialized centres and often take years.
• Inherited myopathies are often characterized by spinal rigidity.
• Whole body magnetic resonance imaging is a unique tool to orientate the diagnosis of each inherited myopathy presenting with spinal rigidity.
• Each inherited myopathy in this study has a specific pattern of affected muscles that orientate diagnosis.
• A novel MRI-based algorithm, usable by every radiologist, can help the early diagnosis of these myopathies.



Similar content being viewed by others
Abbreviations
- MAM:
-
Mean of percentages of affected muscle
- mWB-MRI:
-
Muscular whole body magnetic resonance imaging
- RSS:
-
Rigid spine syndrome
References
Emery AE (1991) Population frequencies of inherited neuromuscular diseases–a world survey. Neuromuscul Disord 1:19–29
North KN, Wang CH, Clarke N et al (2014) Approach to the diagnosis of congenital myopathies. Neuromuscul Disord 24:97–116
Bönnemann CG, Wang CH, Quijano-Roy S et al (2014) Diagnostic approach to the congenital muscular dystrophies. Neuromuscul Disord 24:289–311
Ferreiro A, Quijano-Roy S, Pichereau C et al (2002) Mutations of the selenoprotein N gene, which is implicated in rigid spine muscular dystrophy, cause the classical phenotype of multiminicore disease: reassessing the nosology of early-onset myopathies. Am J Hum Genet 71:739–749
Quijano-Roy S, Avila-Smirnow D, Carlier RY, WB-MRI muscle study group (2012) Whole body muscle MRI protocol: pattern recognition in early onset NM disorders. Neuromuscul Disord 22(Suppl 2):S68–S84
Wattjes MP, Kley RA, Fischer D (2010) Neuromuscular imaging in inherited muscle diseases. Eur Radiol 20:2247–2460
Mercuri E, Pichiecchio A, Allsop J, Messina S, Pane M, Muntoni F (2007) Muscle MRI in inherited neuromuscular disorders: past, present, and future. J Magn Reson Imaging 25:433–440
Hankiewicz K, Carlier RY, Lazaro L et al (2015) Whole-body muscle magnetic resonance imaging in SEPN1-related myopathy shows a homogeneous and recognizable pattern. Muscle Nerve 52:728–735
Gómez-Andrés D, Dabaj I, Mompoint D et al (2016) Pediatric laminopathies: whole-body magnetic resonance imaging fingerprint and comparison with SEPN1 myopathy. Muscle Nerve 54:192–202
Carboni N, Mura M, Marrosu G et al (2008) Muscle MRI findings in patients with an apparently exclusive cardiac phenotype due to a novel LMNA gene mutation. Neuromuscul Disord 18:291–298
Mercuri E, Pichiecchio A, Counsell S et al (2002) A short protocol for muscle MRI in children with muscular dystrophies. Eur J Paediatr Neurol 6:305–307
Fischer D, Bonati U, Wattjes MP (2016) Recent developments in muscle imaging of neuromuscular disorders. Current Opin Neurol 29:614–620
Dubowitz V (1973) Rigid spine syndrome: a muscle syndrome in search of a name. Proc R Soc Med 66:219–220
Kishnani PS, Corzo D, Nicolino M et al (2007) Recombinant human acid [alpha]-glucosidase: major clinical benefits in infantile-onset Pompe disease. Neurology 68:99–109
Wu S, Ibarra MCA, Malicdan MCV et al (2006) Central core disease is due to RYR1 mutations in more than 90% of patients. Brain 129:1470–1480
Wilmshurst JM, Lillis S, Zhou H et al (2010) RYR1 mutations are a common cause of congenital myopathies with central nuclei. Ann Neurol 68:717–726
Baker NL, Mörgelin M, Peat R et al (2005) Dominant Collagen VI mutations are a common cause of Ullrich congenital muscular dystrophy. Hum Mol Genet 14:279–293
Quijano-Roy S, Mbieleu B, Bönnemann CG et al (2008) De novo LMNA mutations cause a new form of congenital muscular dystrophy. Ann Neurol 64:177–186
Cagliani R, Fruguglietti ME, Berardinelli A et al (2011) New molecular findings in congenital myopathies due to selenoprotein N gene mutations. J Neurol Sci 300:107–113
Moghadaszadeh B, Petit N, Jaillard C et al (2001) Mutations in SEPN1 cause congenital muscular dystrophy with spinal rigidity and restrictive respiratory syndrome. Nat Genet 29:17–18
Turner C, Mein R, Shape C, Love DR (2015) Merosin-deficient congenital muscular dystrophy: a novel homozygous mutation in the laminin-2 gene. J Clin Neurosci 22:1983–1985
Tajsharghi H, Thornell L-E, Lindberg C, Lindvall B, Henriksson K-G, Oldfors A (2003) Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol 54:494–500
Hollingsworth KG, de Sousa PL, Straub V, Carlier PG (2012) Towards harmonization of protocols for MRI outcome measures in skeletal muscle studies: consensus recommendations from two TREAT-NMD NMR workshops, 2 May 2010, Stockholm, Sweden, 1-2 October 2009, Paris, France. Neuromuscul Disord 22:S54–S67
Mercuri E, Counsell S, Allsop J et al (2002) Selective muscle involvement on magnetic resonance imaging in autosomal dominant Emery-Dreifuss muscular dystrophy. Neuropediatrics 33:10–14
Fleiss JL (1971) Measuring nominal scale agreement among many raters. Psychol Bull 76:378–382
Cohen J (1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20:37–46
Lilliefors HW (1967) On the Kolmogorov-Smirnov test for normality with mean and variance unknown. J Am Stat Assoc 62:399–402
Mann HB, Whitney DR (1947) On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat 50–60
Wilcoxon F (1945) Individual comparisons by ranking methods. Biom Bull 1:80
Mercuri E, Clements E, Offiah A et al (2010) Muscle magnetic resonance imaging involvement in muscular dystrophies with rigidity of the spine. Ann Neurol 67:201–208
Jungbluth H, Davis MR, Müller C et al (2004) Magnetic resonance imaging of muscle in congenital myopathies associated with RYR1 mutations. Neuromuscul Disord 14:785–790
Díaz-Manera J, Alejaldre A, González L et al (2016) Muscle imaging in muscle dystrophies produced by mutations in the EMD and LMNA genes. Neuromuscul Disord 26:33–40
Carlier RY, Laforet P, Wary C et al (2011) Whole-body muscle MRI in 20 patients suffering from late onset Pompe disease: involvement patterns. Neuromuscul Disord 21:791–799
Lamer S, Carlier RY, Pinard JM et al (1998) Congenital muscular dystrophy: use of brain MR imaging findings to predict merosin deficiency. Radiology 206:811–816
Schessl J, Medne L, Hu Y et al (2007) MRI in DNM2-related centronuclear myopathy: evidence for highly selective muscle involvement. Neuromuscul Disord 17:28–32
Catteruccia M, Fattori F, Codemo V et al (2013) Centronuclear myopathy related to dynamin 2 mutations: clinical, morphological, muscle imaging and genetic features of an Italian cohort. Neuromuscul Disord 23:229–238
Topaloglu H, Gögüs S, Yalaz K, Kücükali T, Serdaroglu A (1994) Two siblings with nemaline myopathy presenting with rigid spine syndrome. Neuromuscul Disord 4:263–267
Sarnat HB (1995) Siblings with rigid spine syndrome and nemaline rod myopathy, a unique association. Neuromuscul Disord 5:351–352
O’Grady GL, Best HA, Oates EC et al (2015) Recessive ACTA1 variant causes congenital muscular dystrophy with rigid spine. Eur J Hum Genet 23:883–886
Norwood FL, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V (2009) Prevalence of genetic muscle disease in North England: in-depth analysis of a muscle clinic population. Brain 132:3175–3186
Worman HJ, Bonne G (2007) “Laminopathies”: a wide spectrum of human diseases. Exp Cell Res 313:2121–2133
Acknowledgements
We thank the patients and their families for their invaluable contributions. We also thank Nathaniel Bern for his help with statistics, Pr Dominique Berrebi, Dr Lea Chiche and Annaelle Chetrit, Dr Jessica Beaziz for their advice. We thank Dr Joseph Benzakoun, Dr Wagih Ben Hassen, Dr Jeffery Zhou, Dr Anh Minh and Dr Corentin Provost for their help with making up the validation group.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Mickael Tordjman
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Nathaniel Bern kindly provided statistical advice for this manuscript. One of the authors has significant statistical expertise: Moustafa Biyoukar.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional review board approval was obtained.
Methodology
• retrospective
• diagnostic or prognostic study/observational
• multicentre study
Electronic supplementary material
ESM 1
(DOCX 17032 kb)
Rights and permissions
About this article
Cite this article
Tordjman, M., Dabaj, I., Laforet, P. et al. Muscular MRI-based algorithm to differentiate inherited myopathies presenting with spinal rigidity. Eur Radiol 28, 5293–5303 (2018). https://doi.org/10.1007/s00330-018-5472-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5472-5