Abstract
Objectives
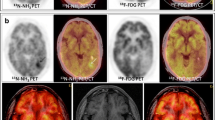
To compare the diagnostic performance of amide proton transfer (APT) imaging and 11-C methionine positron emission tomography (MET-PET) for in vivo molecular imaging of protein metabolism in post-treatment gliomas.
Materials and methods
This study included 43 patients (12 low and 31 high grade) with post-treatment gliomas who underwent both APT and MET-PET imaging within 3 weeks. APT-weighted voxel values and semi-quantitative tumour-to-normal ratios (TNR) were obtained from tumour portions. The voxel-wise relationships between TNR and APT were assessed. The diagnostic performance for recurrence of high-grade gliomas was calculated, using the area under the receiver operating characteristic curve (AUC) with maximum (TNRmax and APTmax) and 90% histogram values (TNR90 and APT90).
Results
A moderate positive correlation between TNR and APT was found in low-grade recurrences (r = 0.47, p < 0.001), but not in high-grade ones (r = −0.24, p < 0.001). For distinguishing recurrence in post-treatment high-grade gliomas, APTmax (AUC, 0.88) and APT90 (AUC, 0.78–0.83) had a similar to better diagnostic performance than TNRmax (AUC, 0.71, p = 0.08) or TNR90 (AUC, 0.53–0.59, p = 0.01–0.05).
Conclusions
In post-treatment high-grade gliomas, APT provides different regional information to MET-PET and provides higher diagnostic performance. This difference needs to be considered when using APT or MET-PET as a surrogate marker for tumour protein metabolism.
Key Points
• APT and TNR values in low-grade recurrence showed a moderate voxel-wise correlation.
• APT and TNR demonstrated regional differences in post-treatment high-grade gliomas.
• APT90 showed better diagnostic performance than TNR90 in high-grade recurrence.





Similar content being viewed by others
Abbreviations
- AUC:
-
Area under the receiver-operating characteristic curve
- APT:
-
Amide proton transfer
- CEST:
-
Chemical exchange saturation transfer
- MET-PET:
-
11-C-methionine positron emission tomography
- RF:
-
Radiofrequency
- ROC:
-
Receiver-operating characteristic
- TNR:
-
Tumour-to-normal ratio
References
Schober O, Duden C, Meyer GJ, Muller JA, Hundeshagen H (1987) Non selective transport of [11C-methyl]-L-and D-methionine into a malignant glioma. Eur J Nucl Med 13:103–105
Smits A, Baumert BG (2011) The clinical value of PET with amino acid tracers for gliomas WHO Grade II. Int J Mol Imaging 2011:372509
Tripathi M, Sharma R, Varshney R et al (2012) Comparison of F-18 FDG and C-11 methionine PET/CT for the evaluation of recurrent primary brain tumors. Clin Nucl Med 37:158–163
Petrirena GJ, Goldman S, Delattre JY (2011) Advances in PET imaging of brain tumors: a referring physician's perspective. Current Opinion in Oncology 23:617–623
Rheims S, Rubi S, Bouvard S et al (2014) Accuracy of distinguishing between dysembryoplastic neuroepithelial tumors and other epileptogenic brain neoplasms with [C-11] methionine PET. Neuro-Oncology 16:1417–1426
Nuutinen J, Sonninen P, Lehikoinen P et al (2000) Radiotherapy treatment planning and long-term follow-up with [(11)C]methionine PET in patients with low-grade astrocytoma. Int J Radiat Oncol Biol Phys 48:43–52
Ribom D, Eriksson A, Hartman M et al (2001) Positron emission tomography (11)C-methionine and survival in patients with low-grade gliomas. Cancer 92:1541–1549
Ullrich RT, Kracht L, Brunn A et al (2009) Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J Nucl Med 50:1962–1968
Pirotte B, Goldman S, Van Bogaert P et al (2005) Integration of [11C]methionine-positron emission tomographic and magnetic resonance imaging for image-guided surgical resection of infiltrative low-grade brain tumors in children. Neurosurgery 57:128–139 discussion 128-139
Jones CK, Schlosser MJ, van Zijl PC, Pomper MG, Golay X, Zhou J (2006) Amide proton transfer imaging of human brain tumors at 3T. Magn Reson Med 56:585–592
van Zijl PC, Yadav NN (2011) Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn Reson Med 65:927–948
Kim J, Wu Y, Guo Y, Zheng H, Sun PZ (2015) A review of optimization and quantification techniques for chemical exchange saturation transfer MRI toward sensitive in vivo imaging. Contrast Media Mol Imaging 10:163–178
Yan K, Fu Z, Yang C et al (2015) Assessing amide proton transfer (APT) MRI contrast origins in 9 L gliosarcoma in the rat brain using proteomic analysis. Mol Imaging Biol 17:479–487
Togao O, Yoshiura T, Keupp J et al (2014) Amide proton transfer imaging of adult diffuse gliomas: correlation with histopathological grades. Neuro Oncol 16:441–448
Park JE, Kim HS, Park KJ, Choi CG, Kim SJ (2015) Histogram analysis of amide proton transfer imaging to identify contrast-enhancing low-grade brain tumor that mimics high-grade tumor: increased accuracy of MR perfusion. Radiology 277:151–161
Togao O, Hiwatashi A, Yamashita K et al (2016) Grading diffuse gliomas without intense contrast enhancement by amide proton transfer MR imaging: comparisons with diffusion- and perfusion-weighted imaging. Eur Radiol
Park JE, Kim HS, Park KJ, Kim SJ, Kim JH, Smith SA (2016) Pre- and posttreatment glioma: comparison of amide proton transfer imaging with MR spectroscopy for biomarkers of tumor proliferation. Radiology 278:514–523
Park KJ, Kim HS, Park JE, Shim WH, Kim SJ, Smith SA (2016) Added value of amide proton transfer imaging to conventional and perfusion MR imaging for evaluating the treatment response of newly diagnosed glioblastoma. Eur Radiol
Doyle WK, Budinger TF, Valk PE, Levin VA, Gutin PH (1987) Differentiation of cerebral radiation necrosis from tumor recurrence by [18F]FDG and 82Rb positron emission tomography. J Comput Assist Tomogr 11:563–570
Sonoda Y, Kumabe T, Takahashi T, Shirane R, Yoshimoto T (1998) Clinical usefulness of 11C-MET PET and 201T1 SPECT for differentiation of recurrent glioma from radiation necrosis. Neurol Med Chir (Tokyo) 38:342–347 discussion 347-348
Ogawa T, Kanno I, Shishido F et al (1991) Clinical value of PET with 18F-fluorodeoxyglucose and L-methyl-11C-methionine for diagnosis of recurrent brain tumor and radiation injury. Acta Radiol 32:197–202
Roelcke U, Radu E, Ametamey S, Pellikka R, Steinbrich W, Leenders KL (1996) Association of rubidium and C-methionine uptake in brain tumors measured by positron emission tomography. J Neurooncol 27:163–171
Heo HY, Lee DH, Zhang Y et al (2017) Insight into the quantitative metrics of chemical exchange saturation transfer (CEST) imaging. Magn Reson Med 77:1853–1865
Terakawa Y, Tsuyuguchi N, Iwai Y et al (2008) Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med 49:694–699
Louis DN, Ohgaki H, Wiestler OD et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Louis DN, Perry A, Reifenberger G et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131:803–820
van den Bent MJ, Wefel JS, Schiff D et al (2011) Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol 12:583–593
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Keupp J, Baltes C, Harvey P, J. van den Brink (2011) Parallel RF transmission based MRI technique for highly sensitive detection of amide proton transfer in the human brain at 3T. In: Proc 19th Annual Meeting ISMRM Montreal
Xiang QS (2006) Two-point water-fat imaging with partially-opposed-phase (POP) acquisition: an asymmetric Dixon method. Magn Reson Med 56:572–584
Eggers H, Brendel B, Duijndam A, Herigault G (2011) Dual-echo Dixon imaging with flexible choice of echo times. Magn Reson Med 65:96–107
Tee YK, Donahue MJ, Harston GW, Payne SJ, Chappell MA (2014) Quantification of amide proton transfer effect pre- and post-gadolinium contrast agent administration. J Magn Reson Imaging 40:832–838
Chung WJ, Kim HS, Kim N, Choi CG, Kim SJ (2013) Recurrent glioblastoma: optimum area under the curve method derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. Radiology 269:561–568
Jeong HK, Han K, Zhou J et al (2017) Characterizing amide proton transfer imaging in haemorrhage brain lesions using 3T MRI. Eur Radiol 27:1577–1584
Ceyssens S, Van Laere K, de Groot T, Goffin J, Bormans G, Mortelmans L (2006) [11C]methionine PET, histopathology, and survival in primary brain tumors and recurrence. AJNR Am J Neuroradiol 27:1432–1437
Puttick S, Bell C, Dowson N, Rose S, Fay M (2015) PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov Today 20:306–317
Takenaka S, Asano Y, Shinoda J et al (2014) Comparison of 11C-Methionine, 11C-Choline, and 18F-Fluorodeoxyglucose-PET for Distinguishing Glioma Recurrence from Radiation Necrosis. Neurologia medico-chirurgica 54:280–289
Togao O, Hiwatashi A, Keupp J et al (2015) Scan-rescan reproducibility of parallel transmission based amide proton transfer imaging of brain tumors. J Magn Reson Imaging 42:1346–1353
Scheidegger R, Wong ET, Alsop DC (2014) Contributors to contrast between glioma and brain tissue in chemical exchange saturation transfer sensitive imaging at 3 Tesla. Neuroimage 99:256–268
Heo HY, Zhang Y, Lee DH, Hong X, Zhou J (2016) Quantitative assessment of amide proton transfer (APT) and nuclear overhauser enhancement (NOE) imaging with extrapolated semi-solid magnetization transfer reference (EMR) signals: Application to a rat glioma model at 4.7 Tesla. Magn Reson Med 75:137–149
Li H, Li K, Zhang XY et al (2015) R1 correction in amide proton transfer imaging: indication of the influence of transcytolemmal water exchange on CEST measurements. NMR Biomed 28:1655–1662
Funding
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIP) (grant no. NRF-2017R1C1B2007258), by a grant from the National R&D Program for Cancer Control, Ministry of Health and Welfare, Republic of Korea (1720030).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Sang Joon Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board of Asan Medical Center approved this study (2017-0528).
Methodology
• retrospective
• cross-sectional study
• performed at one institution
Electronic supplementary material
ESM 1
(DOCX 943 kb)
Rights and permissions
About this article
Cite this article
Park, J.E., Lee, J.Y., Kim, H.S. et al. Amide proton transfer imaging seems to provide higher diagnostic performance in post-treatment high-grade gliomas than methionine positron emission tomography. Eur Radiol 28, 3285–3295 (2018). https://doi.org/10.1007/s00330-018-5341-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-018-5341-2