Abstract
Objectives
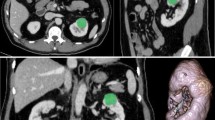
To apply a statistical clustering algorithm to combine information from dynamic contrast-enhanced (DCE) magnetic resonance imaging (MRI) into a single tumour map to distinguish high-grade from low-grade T1b clear cell renal cell carcinoma (ccRCC).
Methods
This prospective, Institutional Review Board -approved, Health Insurance Portability and Accountability Act -compliant study included 18 patients with solid T1b ccRCC who underwent pre-surgical DCE MRI. After statistical clustering of the parametric maps of the transfer constant between the intravascular and extravascular space (K trans), rate constant (K ep ) and initial area under the concentration curve (iAUC) with a fuzzy c-means (FCM) algorithm, each tumour was segmented into three regions (low/medium/high active areas). Percentages of each region and tumour size were compared to tumour grade at histopathology. A decision-tree model was constructed to select the best parameter(s) to predict high-grade ccRCC.
Results
Seven high-grade and 11 low-grade T1b ccRCCs were included. High-grade histology was associated with higher percent high active areas (p = 0.0154) and this was the only feature selected by the decision tree model, which had a diagnostic performance of 78% accuracy, 86% sensitivity, 73% specificity, 67% positive predictive value and 89% negative predictive value.
Conclusions
The FCM integrates multiple DCE-derived parameter maps and identifies tumour regions with unique pharmacokinetic characteristics. Using this approach, a decision tree model using criteria beyond size to predict tumour grade in T1b ccRCCs is proposed.
Key Points
• Tumour size did not correlate with tumour grade in T1b ccRCC.
• Tumour heterogeneity can be analysed using statistical clustering via DCE-MRI parameters.
• High-grade ccRCC has a larger percentage of high active area than low-grade ccRCCs.
• A decision-tree model offers a simple way to differentiate high/low-grade ccRCCs.





Similar content being viewed by others
Abbreviations
- ccRCC:
-
clear-cell renal cell carcinoma
- K trans :
-
transfer constant between the intravascular and extravascular extracellular space
- K ep :
-
rate constant
- Ve :
-
fractional volume of the extravascular extracellular space
- Vp :
-
fractional plasma volume
- iAUC :
-
initial area under the concentration curve
- Lengthmax :
-
maximum length
- AreaROI :
-
tumour area within a region of interest drawn to outline the periphery of the renal mass
- Volest :
-
estimated tumour volume
- FCM:
-
fuzzy c-means
- LAA:
-
low active area
- MAA:
-
medium active area
- HAA:
-
high active area
References
American Cancer Society (2016) Cancer facts and figures 2016. Available at: http://www.cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
Chow WH, Devesa SS, Warren JL, Fraumeni JF Jr (1999) Rising incidence of renal cell cancer in the United States. JAMA 281:1628–1631
Cooperberg MR et al (2008) Decreasing size at diagnosis of stage 1 renal cell carcinoma: analysis from the National Cancer Data Base, 1993 to 2004. J Urol 179:2131–2135
Campbell SC et al (2009) Guideline for management of the clinical T1 renal mass. J Urol 182:1271–1279
Abel EJ et al (2010) Identifying the risk of disease progression after surgery for localized renal cell carcinoma. BJU Int 106:1277–1283
Teloken PE et al (2009) Prognostic impact of histological subtype on surgically treated localized renal cell carcinoma. J Urol 182:2132–2136
Igarashi T et al (2001) The impact of a 4 cm. cutoff point for stratification of T1N0M0 renal cell carcinoma after radical nephrectomy. J Urol 165:1103–1106
Mehrazin R et al (2014) Growth kinetics and short-term outcomes of cT1b and cT2 renal masses under active surveillance. J Urol 192:659–664
Dechet CB et al (2003) Prospective analysis of computerized tomography and needle biopsy with permanent sectioning to determine the nature of solid renal masses in adults. J Urol 169:71–74
Lebret T et al (2007) Percutaneous core biopsy for renal masses: indications, accuracy and results. J Urol 178:1184–1188, discussion 1188
Sun M et al (2012) Treatment management of small renal masses in the 21st century: a paradigm shift. Ann Surg Oncol 19:2380–2387
Sun MR et al (2009) Renal cell carcinoma: dynamic contrast-enhanced MR imaging for differentiation of tumor subtypes—correlation with pathologic findings. Radiology 250:793–802
Chandarana H et al (2012) Histogram analysis of whole-lesion enhancement in differentiating clear cell from papillary subtype of renal cell cancer. Radiology 265:790–798
Notohamiprodjo M et al (2013) Combined diffusion-weighted, blood oxygen level-dependent, and dynamic contrast-enhanced MRI for characterization and differentiation of renal cell carcinoma. Acad Radiol 20:685–693
Zhang Y et al (2016) Tumor vascularity in renal masses: correlation of arterial spin-labeled and dynamic contrast-enhanced magnetic resonance imaging assessments. Clin Genitourin Cancer 14:e25–e36
Chandarana H et al (2013) High temporal resolution 3D gadolinium-enhanced dynamic MR imaging of renal tumors with pharmacokinetic modeling: preliminary observations. J Magn Reson Imaging 38:802–808
Wu WC, Su MY, Chang CC, Tseng WY, Liu KL (2011) Renal perfusion 3-T MR imaging: a comparative study of arterial spin labeling and dynamic contrast-enhanced techniques. Radiology 261:845–853
Notohamiprodjo M et al (2010) Measuring perfusion and permeability in renal cell carcinoma with dynamic contrast-enhanced MRI: a pilot study. J Magn Reson Imaging 31:490–501
Abdel Razek AA, Mousa A, Farouk A, Nabil N (2016) Assessment of semiquantitative parameters of dynamic contrast-enhanced perfusion MR Imaging in differentiation of subtypes of renal cell carcinoma. Pol J Radiol 81:90–94
Cornelis F et al (2015) Multiparametric magnetic resonance imaging for the differentiation of low and high grade clear cell renal carcinoma. Eur Radiol 25:24–31
Yang X et al (2012) Nonrigid registration and classification of the kidneys in 3D dynamic contrast enhanced (DCE) MR images. Proc SPIE Int Soc Opt Eng 8314:83140B
Chang YC et al (2012) Classification of breast mass lesions using model-based analysis of the characteristic kinetic curve derived from fuzzy c-means clustering. Magn Reson Imaging 30:312–322
Shi J et al (2009) Treatment response assessment of breast masses on dynamic contrast-enhanced magnetic resonance scans using fuzzy c-means clustering and level set segmentation. Med Phys 36(2009):5052–5063
Farjam R, Tsien CI, Lawrence TS, Cao Y (2014) DCE-MRI defined subvolumes of a brain metastatic lesion by principle component analysis and fuzzy-c-means clustering for response assessment of radiation therapy. Med Phys 41, 011708. doi:10.1118/1.4842556
Fram EK et al (1987) Rapid calculation of T1 using variable flip angle gradient refocused imaging. Magn Reson Imaging 5:201–208
Periaswamy S, Farid H (2003) Elastic registration in the presence of intensity variations. IEEE Trans Med Imaging 22:865–874
Parker GJ et al (2006) Experimentally-derived functional form for a population-averaged high-temporal-resolution arterial input function for dynamic contrast-enhanced MRI. Magn Reson Med 56:993–1000
Evelhoch JL (1999) Key factors in the acquisition of contrast kinetic data for oncology. J Magn Reson Imaging 10:254–259
O'Connor JPB, Jackson A, Parker GJM, Roberts C, Jayson GC (2012) Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat Rev Clin Oncol 9:167–177
Bezdek JC (2012) Pattern recognition with fuzzy objective function algorithms. Springer, New York
Breiman L, Friedman JH, Olshen RA, Stone CJ (1984) Classification and regression trees. Wadsworth, Belmont
Efron B (1986) Jackknife, bootstrap and other resampling methods in regression analysis. Ann Stat 14:1301–1304
Stephenson AJ, Hakimi AA, Snyder ME, Russo P (2004) Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol 171:130–134
Uzzo RG, Novick AC (2001) Nephron sparing surgery for renal tumors: indications, techniques and outcomes. J Urol 166:6–18
Volpe A et al (2011) Contemporary management of small renal masses. Eur Urol 60:501–515
Mally AD, Gayed B, Averch T, Davies B (2012) The current role of percutaneous biopsy of renal masses. Can J Urol 19:6243–6249
Phe V, Yates DR, Renard-Penna R, Cussenot O, Roupret M (2012) Is there a contemporary role for percutaneous needle biopsy in the era of small renal masses? BJU Int 109:867–872
Gerlinger M et al (2012) Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366:883–892
Pedrosa I et al (2008) MR classification of renal masses with pathologic correlation. Eur Radiol 18:365–375
Yang X, Knopp MV (2011) Quantifying tumor vascular heterogeneity with dynamic contrast-enhanced magnetic resonance imaging: a review. J Biomed Biotechnol 2011:732848
Sinha S et al (1997) Multifeature analysis of Gd-enhanced MR images of breast lesions. J Magn Reson Imaging 7:1016–1026
Chen W, Giger ML, Li H, Bick U, Newstead GM (2007) Volumetric texture analysis of breast lesions on contrast-enhanced magnetic resonance images. Magn Reson Med 58:562–571
O'Sullivan F, Roy S, Eary J (2003) A statistical measure of tissue heterogeneity with application to 3D PET sarcoma data. Biostatistics 4:433–448
O'Sullivan F, Roy S, O'Sullivan J, Vernon C, Eary J (2005) Incorporation of tumor shape into an assessment of spatial heterogeneity for human sarcomas imaged with FDG-PET. Biostatistics 6:293–301
Shin TY et al (2014) Assessing the anatomical characteristics of renal masses has a limited effect on the prediction of pathological outcomes in solid, enhancing, small renal masses: results using the PADUA classification system. BJU Int 113:754–761
Yuan Q et al (2016) Intratumor heterogeneity of perfusion and diffusion in clear-cell renal cell carcinoma: correlation with tumor cellularity. Clin Genitourin Cancer 14:e585–e594-T1b
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Ivan Pedrosa.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
This study has received funding by the NIH grants no. P50CA196516 and no. 5RO1CA154475.
Statistics and biometry
One of the authors has significant statistical expertise.
Ethical approval
Institutional Review Board approval was obtained.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Study subjects or cohorts overlap
Some study subjects or cohorts have been previously reported by Yuan et al. [46].
Methodology
Prospective, cross-sectional study; observational. Performed at one institution.
Rights and permissions
About this article
Cite this article
Xi, Y., Yuan, Q., Zhang, Y. et al. Statistical clustering of parametric maps from dynamic contrast enhanced MRI and an associated decision tree model for non-invasive tumour grading of T1b solid clear cell renal cell carcinoma. Eur Radiol 28, 124–132 (2018). https://doi.org/10.1007/s00330-017-4925-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4925-6