Abstract
Objectives
T1 relaxometry is a promising tool for the assessment of microstructural changes during brain ageing. Previous cross-sectional studies demonstrated increasing T1 values in white and decreasing T1 values in grey matter over the lifetime. However, these findings have not yet been confirmed on the basis of a longitudinal study. In this longitudinal study over 7 years, T1 relaxometry was used to investigate the dynamics of age-related microstructural changes in older healthy subjects.
Methods
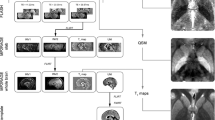
T1 mapping was performed in 17 healthy subjects (range 51–77 years) at baseline and after 7 years. Advanced cortical and white matter segmentation was used to determine mean T1 values in the cortex and white matter.
Results
The analysis revealed a decrease of mean cortical T1 values over 7 years, the rate of T1 reduction being more prominent in subjects with higher age. T1 decreases were predominantly localized in the lateral frontal, parietal and temporal cortex. In contrast, mean white matter T1 values remained stable.
Conclusions
T1 mapping is shown to be sensitive to age-related microstructural changes in healthy ageing subjects in a longitudinal setting. Data of a cohort in late adulthood and the senescence period demonstrate a decrease of cortical T1 values over 7 years, most likely reflecting decreasing water content and increased iron concentrations.
Key Points
• T1 mapping is sensitive to age-related microstructural changes in a longitudinal setting.
• T1 decreases were predominantly localized in the lateral frontal, parietal and temporal cortex.
• The rate of T1 reduction was more prominent in subjects with higher age.
• These changes most likely reflect decreasing cortical water and increasing iron concentrations.





Similar content being viewed by others
Abbreviations
- CSF:
-
Cerebrospinal fluid
- FSL:
-
FMRIB Software Library
- GE:
-
Gradient echo
- GM:
-
Grey matter
- MNI:
-
Montreal Neurological Institute
- MP-RAGE:
-
Magnetization-prepared rapid gradient-echo
- MRI:
-
Magnetic resonance imaging
- qMRI:
-
Quantitative magnetic resonance imaging
- RF:
-
Radio frequency
- ROI:
-
Region of interest
- WM:
-
White matter
References
Connell J, Brazier J, O’Cathain A, Lloyd-Jones M, Paisley S (2012) Quality of life of people with mental health problems: a synthesis of qualitative research. Health Qual Life Outcomes 10:138
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB (2014) What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog Neurobiol 117:20–40
Thambisetty M, Wan J, Carass A, An Y, Prince JL, Resnick SM (2010) Longitudinal changes in cortical thickness associated with normal aging. NeuroImage 52:1215–1223
Long X, Liao W, Jiang C, Liang D, Qiu B, Zhang L (2012) Healthy aging: an automatic analysis of global and regional morphological alterations of human brain. Acad Radiol 19:785–793
Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR et al (2012) Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging 33:617.e1-9
Tofts P (ed) (2003) Quantitative MRI of the brain: measuring changes caused by disease. Wiley, Chichester
Deoni SCL (2010) Quantitative relaxometry of the brain. Top Magn Reson Imaging 21:101–113
Callaghan MF, Freund P, Draganski B, Anderson E, Cappelletti M, Chowdhury R et al (2014) Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol Aging 35:1862–1872
Lutti A, Dick F, Sereno MI, Weiskopf N (2014) Using high-resolution quantitative mapping of R1 as an index of cortical myelination. NeuroImage 93:176–188
Fatouros PP, Marmarou A, Kraft KA, Inao S, Schwarz FP (1991) In vivo brain water determination by T1 measurements: effect of total water content, hydration fraction, and field strength. Magn Reson Med 17:402–413
Rooney WD, Johnson G, Li X, Cohen ER, Kim S-G, Ugurbil K et al (2007) Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn Reson Med 57:308–318
Gelman N, Ewing JR, Gorell JM, Spickler EM, Solomon EG (2001) Interregional variation of longitudinal relaxation rates in human brain at 3.0 T: relation to estimated iron and water contents. Magn Reson Med 45:71–79
Pakkenberg B, Pelvig D, Marner L, Bundgaard MJ, Gundersen HJG, Nyengaard JR et al (2003) Aging and the human neocortex. Exp Gerontol 38:95–99
Freeman SH, Kandel R, Cruz L, Rozkalne A, Newell K, Frosch MP et al (2008) Preservation of neuronal number despite age-related cortical brain atrophy in elderly subjects without Alzheimer disease. J Neuropathol Exp Neurol 67:1205–1212
Neeb H, Zilles K, Shah NJ (2006) Fully-automated detection of cerebral water content changes: study of age- and gender-related H2O patterns with quantitative MRI. NeuroImage 29:910–922
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Steen RG, Gronemeyer SA, Taylor JS (1995) Age-related changes in proton T1 values of normal human brain. J Magn Reson Imaging 5:43–48
Cho S, Jones D, Reddick WE, Ogg RJ, Steen RG (1997) Establishing norms for age-related changes in proton T1 of human brain tissue in vivo. Magn Reson Imaging 15:1133–1143
Saito N, Sakai O, Ozonoff A, Jara H (2009) Relaxo-volumetric multispectral quantitative magnetic resonance imaging of the brain over the human lifespan: global and regional aging patterns. Magn Reson Imaging 27:895–906
Suzuki S, Sakai O, Jara H (2006) Combined volumetric T1, T2 and secular-T2 quantitative MRI of the brain: age-related global changes (preliminary results). Magn Reson Imaging 24:877–887
Wahlund LO, Agartz I, Almqvist O, Basun H, Forssell L, Sääf J et al (1990) The brain in healthy aged individuals: MR imaging. Radiology 174:675–679
Breger RK, Yetkin FZ, Fischer ME, Papke RA, Haughton VM, Rimm AA (1991) T1 and T2 in the cerebrum: correlation with age, gender, and demographic factors. Radiology 181:545–547
Papadopoulos K, Tozer DJ, Fisniku L, Altmann DR, Davies G, Rashid W et al (2010) TI-relaxation time changes over five years in relapsing-remitting multiple sclerosis. Mult Scler 16:427–433
Preibisch C, Deichmann R (2009) Influence of RF spoiling on the stability and accuracy of T1 mapping based on spoiled FLASH with varying flip angles. Magn Reson Med 61:125–135
Gracien R-M, Reitz SC, Hof SM, Fleischer V, Zimmermann H, Droby A et al (2015) Changes and variability of proton density and T1 relaxation times in early multiple sclerosis: MRI markers of neuronal damage in the cerebral cortex. Eur Rad. doi:10.1007/s00330-015-4072-x
Baudrexel S, Nürnberger L, Rüb U, Seifried C, Klein JC, Deller T et al (2010) Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. NeuroImage 51:512–520
Deoni SCL, Peters TM, Rutt BK (2005) High-resolution T1 and T2 mapping of the brain in a clinically acceptable time with DESPOT1 and DESPOT2. Magn Reson Med 53:237–241
Yarnykh VL (2007) Actual flip-angle imaging in the pulsed steady state: a method for rapid three-dimensional mapping of the transmitted radiofrequency field. Magn Reson Med 57:192–200
Nöth U, Hattingen E, Bähr O, Tichy J, Deichmann R (2015) Improved visibility of brain tumors in synthetic MP-RAGE anatomies with pure T1 weighting. NMR Biomed 28:818–830
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23(Suppl 1):19
Zhang Y, Brady M, Smith S (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging 20:45–57
Gracien RM, Jurcoane A, Wagner M, Reitz SC, Mayer C, Volz S et al (2016) Multimodal quantitative MRI assessment of cortical damage in relapsing-remitting multiple sclerosis. J Magn Reson Imaging. doi:10.1002/jmri.25297
Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE (2014) Permutation inference for the general linear model. NeuroImage 92:381–397
Rorden C, Brett M (2000) Stereotaxic display of brain lesions. Behav Neurol 12:191–200
Dale AM, Fischl B, Sereno MI (1999) Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194
Fischl B, Sereno MI, Dale AM (1999) Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage 9:195–207
Reuter M, Schmansky NJ, Rosas HD, Fischl B (2012) Within-subject template estimation for unbiased longitudinal image analysis. NeuroImage 61:1402–1418
Oros-Peusquens AM, Laurila M, Shah NJ (2008) Magnetic field dependence of the distribution of NMR relaxation times in the living human brain. Magn Reson Mater Phys 21:131–147
Ogg RJ, Steen RG (1998) Age-related changes in brain T1 are correlated with iron concentration. Magn Reson Med 40:749–753
Sedlacik J, Boelmans K, Löbel U, Holst B, Siemonsen S, Fiehler J (2014) Reversible, irreversible and effective transverse relaxation rates in normal aging brain at 3T. NeuroImage 84:1032–1041
Ordidge RJ, Gorell JM, Deniau JC, Knight RA, Helpern JA (1994) Assessment of relative brain iron concentrations using T2-weighted and T2*-weighted MRI at 3 Tesla. Magn Reson Med 32:335–341
Connor JR, Menzies SL, St Martin SM, Mufson EJ (1990) Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res 27:595–611
Koenig SH, Brown RD, Spiller M, Lundbom N (1990) Relaxometry of brain: why white matter appears bright in MRI. Magn Reson Med 14:482–495
Koenig SH (1991) Cholesterol of myelin is the determinant of gray-white contrast in MRI of brain. Magn Reson Med 20:285–291
Lintl P, Braak H (1983) Loss of intracortical myelinated fibers: a distinctive age-related alteration in the human striate area. Acta Neuropathol 61:178–182
Dinse J, Härtwich N, Waehnert MD, Tardif CL, Schäfer A, Geyer S et al (2015) A cytoarchitecture-driven myelin model reveals area-specific signatures in human primary and secondary areas using ultra-high resolution in-vivo brain MRI. NeuroImage 114:71–87
Bock NA, Kocharyan A, Liu JV, Silva AC (2009) Visualizing the entire cortical myelination pattern in marmosets with magnetic resonance imaging. J Neurosci Methods 185:15–22
Andersen C (1997) In vivo estimation of water content in cerebral white matter of brain tumour patients and normal individuals: towards a quantitative brain oedema definition. Acta Neurochir (Wein) 139:249–255, discussion 255–6
Fjell AM, McEvoy L, Holland D, Dale AM, Walhovd KB (2013) Brain changes in older adults at very low risk for Alzheimer’s disease. J Neurosci 33:8237–8242
Fjell AM, Walhovd KB (2010) Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci 21:187–221
Acknowledgments
The scientific guarantor of this publication is Simon Baudrexel. The authors of this manuscript declare no relationships with any companies relevant to this study:
Dr R.M. Gracien has received travel funding from Roche.
Dr L. Nürnberger has received travel funding from TEVA, Allergan and Dysport.
Dr P. Hok was supported by the German Academic Exchange Service (DAAD).
Dr Rüb has received funding from Dr. Senckenbergische Stiftung, Frankfurt/Main, Germany.
Dr H. Steinmetz has received speaker’s honoraria from Bayer, Sanofi and Boehringer Ingelheim.
Dr R. Hilker-Roggendorf has received speaker honoraria from Medtronic, Orion, GlaxoSmithKline, TEVA, Cephalon, Solvay, Desitin, and Boehringer Ingelheim as well as travel funding from Medtronic and Cephalon. He serves or has served on a scientific advisory board for Cephalon and has received research funding from the Deutsche Parkinson Vereinigung (dPV), Bundesministerium für Bildung und Forschung and Goethe-University Frankfurt.
Dr J.C. Klein received speaker honoraria and travel reimbursement from Medtronic, AstraZeneca, Abbott Laboratories and AbbVie.
Dr R. Deichmann received compensation as a Consultant for MR scanner procurement by the Wellcome Trust Centre for Neuroimaging, UCL, London, UK.
Dr S. Baudrexel has received research funding from the Bundesministerium für Bildung und Forschung and travel funding from UCB Pharma.
This study has received funding by the Bundesministerium für Bildung und Forschung [DLR 01GO0203; Brain Imaging Center Frankfurt] and the Deutsche Forschungsgemeinschaft [DFG CRC-TR 128; Dr Deichmann].
One of the authors has significant statistical expertise.
Institutional review board approval was obtained.
Written informed consent was obtained from all subjects in this study.
Data from some study subjects have been previously reported in Baudrexel S, Nürnberger L et al. Quantitative mapping of T1 and T2* discloses nigral and brainstem pathology in early Parkinson’s disease. NeuroImage 2010; 51(2):512–20.
Methodology: prospective, observational study performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
René-Maxime Gracien and Lucas Nürnberger contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Top T1 data sets for a representative subject in 2008 on the left side and 2015 on the right side (z = 19.00). Bottom the respective normalized histograms in 2008 (left) and 2015 (right). The grey matter peak has been marked with a red line (JPG 2190 kb)
Rights and permissions
About this article
Cite this article
Gracien, RM., Nürnberger, L., Hok, P. et al. Evaluation of brain ageing: a quantitative longitudinal MRI study over 7 years. Eur Radiol 27, 1568–1576 (2017). https://doi.org/10.1007/s00330-016-4485-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4485-1