Abstract
Objectives
To assess the potential value of preoperative 18F-FDG PET to predict postoperative recurrence of solitary localized primary gastrointestinal stromal tumour (GIST) after radical resection.
Methods
A total of 46 patients with primary GIST who received preoperative 18F-FDG PET and underwent complete resection without neoadjuvant therapy were retrospectively studied. PET findings, including ring-shaped uptake and intense uptake, were compared with Joensuu risk grades using Fisher’s exact test. The prognostic value of the preoperative clinico-imaging variables—age ≥60 years, male, ring-shaped uptake, intense uptake, tumour size >5 cm, heterogeneous CT attenuation and lower gastrointestinal origin—and Joensuu high risk for recurrence-free survival was evaluated using log-rank test and multivariate Cox regression analysis.
Results
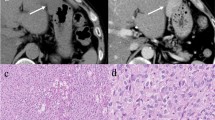
Ring-shaped uptake and intense uptake were significantly associated with Joensuu high risk. Univariate analysis showed that ring-shaped uptake, intense uptake, size >5 cm and Joensuu high risk were significantly associated with inferior recurrence-free survival. Multivariate analysis showed that ring-shaped uptake (P = 0.004) and Joensuu high risk (P = 0.021) were independent adverse prognostic factors of postoperative recurrence.
Conclusions
Ring-shaped uptake on preoperative 18F-FDG PET may be a potential predictor of postoperative tumour recurrence of localized primary GISTs.
Key points
• Clinical course of resectable solitary localized primary GISTs varies widely.
• Ring-shaped uptake is an independent adverse prognostic factor of postoperative recurrence.
• Preoperative 18 F-FDG PET may help predict postoperative recurrence of GISTs.





Similar content being viewed by others
References
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med 130:1466–1478
Hirota S, Isozaki K, Moriyama Y et al (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279:577–580
Demetri GD, von Mehren M, Antonescu CR et al (2010) NCCN Task Force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw 8(Suppl 2):S1–S41
DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231:51–58
Dematteo RP, Ballman KV, Antonescu CR et al (2009) Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, double-blind, placebo-controlled trial. Lancet 373:1097–1104
Joensuu H, Eriksson M, Sundby Hall K et al (2012) One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA 307:1265–1272
ESMO/European Sarcoma Network Working Group (2012) Gastrointestinal stromal tumors: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 23(Suppl 7):vii49–vii55
Eisenberg BL, Smith KD (2011) Adjuvant and neoadjuvant therapy for primary GIST. Cancer Chemother Pharmacol 67(Suppl 1):S3–S8
Jiang Y, Ming L, Montero AJ, Kimchi E, Nikfarjam M, Staveley-O’Carroll KF (2008) Optimizing imatinib mesylate treatment in gastrointestinal stromal tumors. Gastrointest Cancer Res 2:245–250
Andtbacka RH, Ng CS, Scaife CL et al (2007) Surgical resection of gastrointestinal stromal tumors after treatment with imatinib. Ann Surg Oncol 14:14–24
Eisenberg BL, Harris J, Blanke CD et al (2009) Phase II trial of neoadjuvant/adjuvant imatinib mesylate (IM) for advanced primary and metastatic/recurrent operable gastrointestinal stromal tumor (GIST): early results of RTOG 0132/ACRIN 6665. J Surg Oncol 99:42–47
Bonvalot S, Eldweny H, Péchoux CL et al (2006) Impact of surgery on advanced gastrointestinal stromal tumors (GIST) in the imatinib era. Ann Surg Oncol 13:1596–1603
Xu J, Ling TL, Wang M, Zhao WY, Cao H (2015) Preoperative imatinib treatment in patients with advanced gastrointestinal stromal tumors: patient experiences and systematic review of 563 patients. Int Surg 100:860–869
Hecker A, Hecker B, Bassaly B et al (2010) Dramatic regression and bleeding of a duodenal GIST during preoperative imatinib therapy: case report and review. World J Surg Oncol 8:47
Fletcher CD, Berman JJ, Corless C et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol 33:459–565
Joensuu H (2008) Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 39:1411–1419
Rutkowski P, Bylina E, Wozniak A et al (2011) Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol 37:890–896
Gold JS, Gönen M, Gutiérrez A et al (2009) Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol 10:1045–1052
Sepe PS, Moparty B, Pitman MB, Saltzman JR, Brugge WR (2009) EUS-guided FNA for the diagnosis of GI stromal cell tumors: sensitivity and cytologic yield. Gastrointest Endosc 70:254–261
Nishida T, Hirota S, Yanagisawa A et al (2008) Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan: english version. Int J Clin Oncol 13:416–430
Van den Abbeele AD (2008) The lessons of GIST-PET and PET/CT: a new paradigm for imaging. Oncologist 13(Suppl 2):8–13
Langer C, Gunawan B, Schüler P, Huber W, Füzesi L, Becker H (2003) Prognostic factors influencing surgical management and outcome of gastrointestinal stromal tumours. Br J Surg 90:332–339
Aparicio T, Boige V, Sabourin JC et al (2004) Prognostic factors after surgery of primary resectable gastrointestinal stromal tumours. Eur J Surg Oncol 30:1098–1103
Ryu MH, Kang YK, Jang SJ et al (2007) Prognostic significance of p53 gene mutations and protein overexpression in localized gastrointestinal stromal tumours. Histopathology 51:379–389
Kim TW, Lee H, Kang YK et al (2004) Prognostic significance of c-kit mutation in localized gastrointestinal stromal tumors. Clin Cancer Res 10:3076–3081
Tateishi U, Hasegawa T, Satake M, Moriyama N (2003) Gastrointestinal stromal tumor. Correlation of computed tomography findings with tumor grade and mortality. J Comput Assist Tomogr 27:792–798
Dematteo RP, Gold JS, Saran L et al (2008) Tumor mitotic rate, size, and location independently predict recurrence after resection of primary gastrointestinal stromal tumor (GIST). Cancer 112:608–615
Bell SW, Kempson RL, Hendrickson MR (1994) Problematic uterine smooth muscle neoplasms. A clinicopathologic study of 213 cases. Am J Surg Pathol 18:535–558
Tryggvason G, Gíslason HG, Magnússon MK, Jónasson JG (2005) Gastrointestinal stromal tumors in Iceland, 1990-2003: the Icelandic GIST study, a population-based incidence and pathologic risk stratification study. Int J Cancer 117:289–293
Unalp HR, Derici H, Kamer E, Bozdag AD, Tarcan E, Onal MA (2009) Gastrointestinal stromal tumours: outcomes of surgical management and analysis of prognostic variables. Can J Surg 52:31–38
Singer S, Rubin BP, Lux ML et al (2002) Prognostic value of Kit mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 20:3898–3905
Kamiyama Y, Aihara R, Nakabayashi T et al (2005) 18F-fluorodeoxyglucose positron emission tomography: useful technique for predicting malignant potential of gastrointestinal stromal tumors. World J Surg 29:1429–1435
Yamada M, Niwa Y, Matsuura T et al (2007) Gastric GIST malignancy evaluated by 18FDG-PET as compared with EUS-FNA and endoscopic biopsy. Scand J Gastroenterol 42:633–641
Otomi Y, Otsuka H, Morita N et al (2010) Relationship between FDG uptake and the pathological risk category in gastrointestinal stromal tumors. J Med Invest 57:270–274
Park JW, Cho CH, Jeong DS, Chae HD (2011) Role of F-fluoro-2-deoxyglucose positron emission tomography in gastric GIST: predicting malignant potential pre-operatively. J Gastric Cancer 11:173–179
Fendler WP, Chalkidis RP, Ilhan H et al (2015) Evaluation of several FDG PET parameters for prediction of soft tissue tumour grade at primary diagnosis and recurrence. Eur Radiol 25:2214–2221
Van den Abbeele AD, Gatsonis C, de Vries DJ et al (2012) ACRIN 6665/RTOG 0132 phase II trial of neoadjuvant imatinib mesylate for operable malignant gastrointestinal stromal tumor: monitoring with 18F-FDG PET and correlation with genotype and GLUT4 expression. J Nucl Med 53:567–574
Tarn C, Skorobogatko YV, Taguchi T, Eisenberg B, von Mehren M, Godwin AK (2006) Therapeutic effect of imatinib in gastrointestinal stromal tumors: AKT signaling dependent and independent mechanisms. Cancer Res 66:5477–5486
Miettinen M, Sobin LH, Lasoda J (2005) Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol 29:52–68
Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C (2009) Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma 50:1257–1260
Biggi A, Gallamini A, Chauvie S et al (2013) International validation study for interim PET in ABVD-treated, advanced-stage Hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. J Nucl Med 54:683–690
Acknowledgement
The authors thank Dr. Tsuyoshi Terashima (Shiga Medical Center for Adults) for his kind help for data collecting. The scientific guarantor of this publication is Yuji Nakamoto M.D., Ph.D., Department of Diagnostic Imaging and Nuclear Medicine, Kyoto University Hospital.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors (Shiro Tanaka, Ph.D., Department of Pharmacoepidemiology, Graduate School of Medicine and Public Health) has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miyake, K.K., Nakamoto, Y., Mikami, Y. et al. The predictive value of preoperative 18F-fluorodeoxyglucose PET for postoperative recurrence in patients with localized primary gastrointestinal stromal tumour. Eur Radiol 26, 4664–4674 (2016). https://doi.org/10.1007/s00330-016-4242-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4242-5