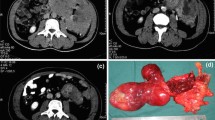
Abstract
Adjuvant therapy for primary GIST has proven benefit in extending disease free survival. Defined risk factors for recurrent disease are based on GIST size, location, and mitotic rate and provide useful guidelines for selecting patients for adjuvant therapy considerations. Neoadjuvant therapy with tyrosine kinase inhibition has potential usefulness in primary GIST, although not yet as standard of care. Advantages can include tumor downsizing to provide opportunity for less morbid surgical resection as well as to decrease risk of intra-op tumor rupture. These theoretical considerations have not been evaluated in large clinical studies.

Similar content being viewed by others
References
Ng EH et al (1992) Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 215(1):68–77
DeMatteo RP et al (2000) Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg 231(1):51–58
Pierie JP et al (2001) The effect of surgery and grade on outcome of gastrointestinal stromal tumors. Arch Surg 136(4):383–389
Crosby JA et al (2001) Malignant gastrointestinal stromal tumors of the small intestine: a review of 50 cases from a prospective database. Ann Surg Oncol 8(1):50–59
Fletcher CD et al (2002) Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol 10(2):81–89
Miettinen M, Lasota J (2006) Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol 23(2):70–83
Takahashi T et al (2007) An enhanced risk-group stratification system for more practical prognostication of clinically malignant gastrointestinal stromal tumors. Int J Clin Oncol 12(5):369–374
Maeda H et al (1992) Requirement of c-kit for development of intestinal pacemaker system. Development 116(2):369–375
Isozaki K et al (1995) Disturbed intestinal movement, bile reflux to the stomach, and deficiency of c-kit-expressing cells in Ws/Ws mutant rats. Gastroenterology 109(2):456–464
Furitsu T et al (1993) Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J Clin Invest 92(4):1736–1744
Nagata H et al (1995) Identification of a point mutation in the catalytic domain of the protooncogene c-kit in peripheral blood mononuclear cells of patients who have mastocytosis with an associated hematologic disorder. Proc Natl Acad Sci USA 92(23):10560–10564
Hirota S et al (1998) Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science 279(5350):577–580
Taniguchi M et al (1999) Effect of c-kit mutation on prognosis of gastrointestinal stromal tumors. Cancer Res 59(17):4297–4300
Joensuu H et al (2001) Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med 344(14):1052–1056
Demetri GD et al (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480
van Oosterom AT et al (2001) Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 358(9291):1421–1423
Blanke CD et al (2008) Long-term results from a randomized phase II trial of standard-versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol 26(4):620–625
Heinrich MC et al (2003) PDGFRA activating mutations in gastrointestinal stromal tumors. Science 299(5607):708–710
Heinrich MC et al (2003) Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol 21(23):4342–4349
Antonescu CR et al (2005) Acquired resistance to imatinib in gastrointestinal stromal tumor occurs through secondary gene mutation. Clin Cancer Res 11(11):4182–4190
Zalcberg JR et al (2005) Outcome of patients with advanced gastro-intestinal stromal tumours crossing over to a daily imatinib dose of 800 mg after progression on 400 mg. Eur J Cancer 41(12):1751–1757
Demetri GD et al (2006) Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet 368(9544):1329–1338
Goodman VL et al (2007) Approval summary: sunitinib for the treatment of imatinib refractory or intolerant gastrointestinal stromal tumors and advanced renal cell carcinoma. Clin Cancer Res 13(5):1367–1373
DeMatteo R, Ballman K, Antonescu C et al (2009) Adjuvant Imatinib Mesylate after resection of localized primary gastrointestinal stromal tumor: a randomized double blind placebo controlled trial. Lancet 373:1097–1104
Blay JY et al (2007) Prospective multicentric randomized phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French Sarcoma Group. J Clin Oncol 25(9):1107–1113
Singer S et al (2002) Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol 20(18):3898–3905
Gold J, Gonen M, Gutierrez J et al (2009) Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localized primary gastroinestinal stromal tumor–a retrospective analysis. Lancet Oncol 10:1045–1052
Casali PG, Jost L, Reichardt P, Schlemmer M, Blay JY (2009) Gastrointestinal stromal tumours: ESMO clinical recommendations for diagnosis, treatment and followup. Ann Oncol 20(4):64–67
NCCN. The NCCN soft tissue sarcoma clinical practice guidelines in oncology. (version 1.2009). (C) 2009 National Comprehensive Cancer Network, Inc
Eisenberg B, Judson I (2004) Surgery and Imatinib in the management of GIST. Emerging approaches to adjuvant and neoadjuvant therapy. Ann Surg Oncol II(5):465–475
Eisenberg B, Harris J, Blanke C, Demetri G et al (2009) Phase II trial of neoadjuvant/adjuvant imatinib mesylate for advanced primary, metastatic/recurrent operable GIST–early results of RTOG. J Surg Oncol 99(1):42–47
Andtbacka R, Ng C, Scaife C, Cormier J et al (2007) Surgical resection of Gastrointestinal Stromal Tumor after treatment with Imatinib. Ann Surg Oncol 14(1):14–24
Raut C, Posner M, Desai J et al (2006) Surgical management of advanced gastrointestinal stromal tumors after treatment with targeted systemic therapy using kinase inhibitors. J Clin Oncol 24:2325–2331
Acknowledgments
Logistical support during submission of this article was provided by Springer Healthcare LLC. This support was funded by Novartis.
Conflict of interest
Dr. Burton L. Eisenberg, MD: Novartis Advisory Board; Dr. Kerrington D. Smith, MD: None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Eisenberg, B.L., Smith, K.D. Adjuvant and neoadjuvant therapy for primary GIST. Cancer Chemother Pharmacol 67 (Suppl 1), 3–8 (2011). https://doi.org/10.1007/s00280-010-1516-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1516-5