Abstract
Objectives
To investigate and correct the temperature dependence of postmortem MR quantification used for soft tissue characterization and differentiation in thoraco-abdominal organs.
Material and methods
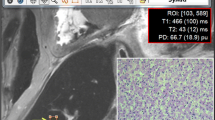
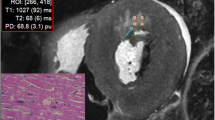
Thirty-five postmortem short axis cardiac 3-T MR examinations were quantified using a quantification sequence. Liver, spleen, left ventricular myocardium, pectoralis muscle and subcutaneous fat were analysed in cardiac short axis images to obtain mean T1, T2 and PD tissue values. The core body temperature was measured using a rectally inserted thermometer. The tissue-specific quantitative values were related to the body core temperature. Equations to correct for temperature differences were generated.
Results
In a 3D plot comprising the combined data of T1, T2 and PD, different organs/tissues could be well differentiated from each other. The quantitative values were influenced by the temperature. T1 in particular exhibited strong temperature dependence. The correction of quantitative values to a temperature of 37 °C resulted in better tissue discrimination.
Conclusion
Postmortem MR quantification is feasible for soft tissue discrimination and characterization of thoraco-abdominal organs. This provides a base for computer-aided diagnosis and detection of tissue lesions. The temperature dependence of the T1 values challenges postmortem MR quantification. Equations to correct for the temperature dependence are provided.
Key points
• Postmortem MR quantification is feasible for soft tissue discrimination and characterization
• Temperature dependence of the T1 values challenges the MR quantification approach
• The results provide the basis for computer-aided postmortem MRI diagnosis
• Diagnostic criteria may also be applied for living patients




Similar content being viewed by others
Abbreviations
- MRI:
-
Magnetic resonance imaging
- PD:
-
Proton density
- pmMRI:
-
Postmortem resonance imaging
- ROI:
-
Region of interest
- T:
-
Tesla
- TE:
-
Echo time
- TR:
-
Repetition time
- T2w:
-
T2-weighted
References
Patriquin L, Kassarjian A, Barish M et al (2001) Postmortem whole-body magnetic resonance imaging as an adjunct to autopsy: preliminary clinical experience. J Magn Reson Imaging 13:277–287
Roberts IS, Benbow EW, Bisset R et al (2003) Accuracy of magnetic resonance imaging in determining cause of sudden death in adults: comparison with conventional autopsy. Histopathology 42:424–430
Dirnhofer R, Jackowski C, Vock P, Potter K, Thali MJ (2006) VIRTOPSY: minimally invasive, imaging-guided virtual autopsy. Radiographics 26:1305–1333
Lundström C, Persson A, Ross S et al (2012) State-of-the-art of visualization in post-mortem imaging. APMIS 120:316–326
Jackowski C, Thali MJ, Buck U et al (2006) Noninvasive estimation of organ weights by postmortem magnetic resonance imaging and multislice computed tomography. Invest Radiol 41:572–578
Jackowski C, Thali MJ, Aghayev E et al (2006) Postmortem imaging of blood and its characteristics using MSCT and MRI. Int J Legal Med 120:233–240
Fitzmaurice GJ, Wishart V, Graham AN (2013) An unexpected mortality following cardiac surgery: a post-mortem diagnosis of cardiac amyloidosis. Gen Thorac Cardiovasc Surg 61:417–421
Aghayev E, Sonnenschein M, Jackowski C et al (2006) Postmortem radiology of fatal hemorrhage: measurements of cross-sectional areas of major blood vessels and volumes of aorta and spleen on MDCT and volumes of heart chambers on MRI. AJR Am J Roentgenol 187:209–215
Kuroiwa Y, Yamashita A, Nishihira K et al (2011) Cardiac rupture in acute myocardial infarction: post-mortem MR imaging. Magn Reson Med Sci 10:255–258
Jackowski C, Christe A, Sonnenschein M, Aghayev E, Thali MJ (2006) Postmortem unenhanced magnetic resonance imaging of myocardial infarction in correlation to histological infarction age characterization. Eur Heart J 27:2459–2467
Jackowski C, Warntjes MJ, Berge J, Bar W, Persson A (2011) Magnetic resonance imaging goes postmortem: noninvasive detection and assessment of myocardial infarction by postmortem MRI. Eur Radiol 21:70–78
Thayyil S, Sebire NJ, Chitty LS et al (2013) Post-mortem MRI versus conventional autopsy in fetuses and children: a prospective validation study. Lancet 382:223–233
Jackowski C, Hofmann K, Schwendener N, Schweitzer W, Keller-Sutter M (2011) Coronary thrombus and peracute myocardial infarction visualized by unenhanced postmortem MRI prior to autopsy. Forensic Sci Int 214:e16–e19
Jackowski C, Schweitzer W, Thali MJ et al (2005) Virtopsy: postmortem imaging of the human heart in situ using MSCT and MRI. Forensic Sci Int 149:11–23
Jackowski C, Schwendener N, Grabherr S, Persson A (2013) Postmortem cardiac 3T magnetic resonance imaging: visualizing the sudden cardiac death? J Am Coll Cardiol 62:617–629
Warntjes JB, Dahlqvist O, Lundberg P (2007) Novel method for rapid, simultaneous T1, T*2, and proton density quantification. Magn Reson Med 57:528–537
Warntjes JB, Leinhard OD, West J, Lundberg P (2008) Rapid magnetic resonance quantification on the brain: optimization for clinical usage. Magn Reson Med 60:320–329
Warntjes MJ, Kihlberg J, Engvall J (2010) Rapid T1 quantification based on 3D phase sensitive inversion recovery. BMC Med Imaging 10:19
Blystad I, Warntjes JB, Smedby O, Landtblom AM, Lundberg P, Larsson EM (2012) Synthetic MRI of the brain in a clinical setting. Acta Radiol 53:1158–1163
Jackowski C, Warntjes MJ, Kihlberg J, Berge J, Thali MJ, Persson A (2011) Quantitative MRI in isotropic spatial resolution for forensic soft tissue documentation. Why and how? J Forensic Sci 56:208–215
Vågberg M, Lindqvist T, Ambarki K et al (2013) Automated determination of brain parenchymal fraction in multiple sclerosis. AJNR Am J Neuroradiol 34:498–504
Synthetic MR (2014) http://www.syntheticmr.com. Accessed 30 Mar 2014
Liu CY, Bluemke DA, Gerstenblith G et al (2014) Reference values of myocardial structure, function, and tissue composition by cardiac magnetic resonance in healthy African-Americans at 3T and their relations to serologic and cardiovascular risk factors. Am J Cardiol 114:789–795
von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA (2013) Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson 15:53
Bazelaire C, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230:652–659
Ding Y, Mason RP, McColl RW et al (2013) Simultaneous measurement of tissue oxygen level-dependent (TOLD) and blood oxygenation level-dependent (BOLD) effects in abdominal tissue oxygenation level studies. J Magn Reson Imaging 38:1230–1236
Pai A, Li X, Majumdar S (2008) A comparative study at 3 T of sequence dependence of T2 quantitation in the knee. Magn Reson Imaging 26:1215–1220
Ljung P, Winskog C, Persson A, Lundström C, Ynnerman A (2006) Full body virtual autopsies using a state-of-the-art volume rendering pipeline. IEEE Trans Vis Comput Graph 12:869–876
Persson A, Lindblom M, Jackowski C (2011) A state-of-the-art pipeline for postmortem CT and MRI visualization: from data acquisition to interactive image interpretation at autopsy. Acta Radiol 52:522–536
Dickinson RJ, Hall AS, Hind AJ, Young IR (1986) Measurement of changes in tissue temperature using MR imaging. J Comput Assist Tomogr 10:468–472
Wlodarczyk W, Hentschel M, Wust P et al (1999) Comparison of four magnetic resonance methods for mapping small temperature changes. Phys Med Biol 44:607–624
Peller M, Kurze V, Loeffler R et al (2003) Hyperthermia induces T1 relaxation and blood flow changes in tumors. A MRI thermometry study in vivo. Magn Reson Imaging 21:545–551
Parker DL, Smith V, Sheldon P, Crooks LE, Fussell L (1983) Temperature distribution measurements in two-dimensional NMR imaging. Med Phys 10:321–325
Youl BD, Hawkins CP, Morris JK, DuBoulay EP, Tofts PS (1992) In vivo T1 values from guinea pig brain depend on body temperature. Magn Reson Med 24:170–173
Haacke ME, Brown RW, Thompson MR, Venkatesh N (1999) Magnetic resonance imaging-physical principles and sequence design. Wiley, New York
Ruder TD, Hatch GM, Siegenthaler L et al (2012) The influence of body temperature on image contrast in post mortem MRI. Eur J Radiol 81:1366–1370
Acknowledgements
The scientific guarantor of this publication is Prof. Christian Jackowski MD, Head of Department, Institute of Forensic Medicine, Bern/Switzerland. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. Beat Kneubuehl, PhD, kindly provided statistical advice for this manuscript. Institutional review board approval was not required because all cases/corpses were investigated by order of the local prosecutors. The prosecutors agreed to research on corpses when the personal data of the deceased persons are treated as strictly confidential. Since we treat all personal data of corpses as confidential we have information from the institutional review board that approval from the review board is not necessary for all our conducted postmortem studies including postmortem imaging. Written informed consent was not required for this study because the subjected studies were deceased persons. None of the study subjects or cohorts have been previously reported. Methodology: prospective, experimental, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zech, WD., Schwendener, N., Persson, A. et al. Temperature dependence of postmortem MR quantification for soft tissue discrimination. Eur Radiol 25, 2381–2389 (2015). https://doi.org/10.1007/s00330-015-3588-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3588-4