Abstract
Left ventricular (LV) function assessment by dual-source computed tomography (DSCT) was compared with the reference standard method using magnetic resonance imaging (MRI). Accurate assessment of LV function is essential for the prediction of prognosis in cardiac disease. Thirty-four patients undergoing DSCT examination of the heart for various clinical indications underwent MRI after DSCT. Short-axis cine images were reconstructed from the DSCT datasets and were analyzed using a dedicated post-processing software-tool to generate global left ventricular function parameters. Five DSCT datasets were considered to be of insufficient image quality. DSCT showed a small overestimation of end-diastolic and end-systolic volumes of 11.0 ml and 3.5 ml, nrespectively. Myocardial mass assessed by DSCT showed an average underestimation of 0.2 g. DSCT showed a small overestimation of LV ejection fraction (LVEF) of 0.4%-point with a Bland-Altman interval of [−8.67 (0.40) 9.48]. Global LV functional parameters calculated from DSCT datasets acquired in daily clinical practice correlated well with MRI and may be considered interchangeable. However, visual assessment of the image quality of the short-axis cine slices should be performed to detect any artifacts in the DSCT data which could influence accuracy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac morbidity and mortality are closely related to cardiac volumes, myocardial mass and global left ventricular (LV) function, expressed as the LV ejection fraction (LVEF). Accurate assessment of these parameters is required for the prediction of prognosis in individual patients as well as in entire cohorts [1–3].
Assessment of LVEF can be performed by a number of different imaging modalities, ranging from echocardiography to direct contrast ventriculography. The current standard of reference for global LV function analysis is short-axis magnetic resonance imaging (MRI), using steady-state free precession (SSFP) sequences [4–6].
The main advantage of MRI is its excellent temporal resolution without exposing the patient to ionizing radiation or iodine-based contrast agents. In addition, MRI does not rely on geometrical assumptions for assessment of LV function parameters. However, MRI is contra-indicated in a substantial number of patients for various reasons, e.g., non-MR-compatible implants or claustrophobia [7].
Furthermore, assessment of coronary artery anatomy using MRI is currently held to be inferior to assessment by CT [8].
For this indication, CT is gaining ground due to several major extensive technological improvements. The most marked development has been the introduction of the multiple-detector computed tomography (MDCT). With ever decreasing gantry rotation times and the introduction of a second X-ray source in dual-source CT (DSCT) technology, the temporal resolution of CT is slowly approaching the temporal resolution obtained with MRI. On the other hand, spatial resolution obtained with contemporary CT techniques already outperforms the spatial resolution of contemporary MRI acquisitions.
Different CT techniques offer different advantages and disadvantages. Sixty-four-slice MDCT offers a high spatial resolution with a relatively low temporal resolution. DSCT combines both a high temporal resolution with a high spatial resolution. Moreover, the image quality of DSCT at higher heart rates is superior to that of conventional MDCT systems.
Since DSCT is used ever more frequently in clinical practice, it is increasingly relevant to assess its diagnostic accuracy for analysis of global LV function parameters, since they can easily be calculated from the raw data of a gated DSCT examination of the heart. A recently published meta-analysis showed that the diagnostic accuracy of CT-derived global LV function parameters has increased with the introduction of more advanced CT systems [9].
Therefore, we sought to investigate the diagnostic accuracy of the new DSCT system.
Materials and methods
Patients
The present study was conducted at the University Medical Center Groningen. The protocol was reviewed and approved by the medical ethics committee. Thirty-four consecutive clinical patients with diverse cardiac disease, who were scheduled for DSCT by their treating physician in routine clinical practice were approached for the study. After the DSCT assessment, the patient underwent an MRI examination of the heart. If LV function modifying interventions, such as coronary interventions or medication changes, were carried out in the time between both acquisitions the patient was excluded.
All patient characteristics are displayed in Table 1. There were no limitations for inclusion with regard to the underlying cardiac disease. All patients in the present study had sinus rhythm, allowing adequate ECG triggering.
DSCT data acquisition
All cardiac CT angiographies were performed on a DSCT system (Somatom Definition, Siemens Medical Solutions, Forcheim, Germany). Retrospective ECG-triggered images were obtained, from the carina to the apex, during one breath-hold. The following scan protocol was used: tube voltage was 120 kV and tube current 410 mAs/rot for both tubes. The gantry rotation time was 0.33 s and the pitch ranged from 0.2 to 0.5 s, adapted automatically to the heart rate. The ECG pulsing window was 20–70% of the RR-interval for all patients. Scanning was performed with a 64 × 0.6-mm collimation. Coronary enhancement was achieved by intravenous injection of 80 ml of non-ionic contrast agent (Iomeron 400 mg/ml, Bracco, Milan, Italy) followed by a saline bolus flush. Bolus timing was performed automatically with a region of interest set in the descending aorta with a threshold of 100 Hounsfield units. For LV function analysis, multiphase reconstructions were made at every 10% of the RR-interval. A mono-segmental reconstruction algorithm was used for all image-reconstructions.
MRI data acquisition
All patients were examined on a 1.5-Tesla MR system (Siemens Magnetom Sonata, Erlangen, Germany) using a 2 × 6 channel body-coil. After single-shot localizer images, a retrospectively gated cine SSFP sequence (TR/TE 57.46/1.10 ms, α 59°, FOV 284 × 350 mm, matrix 125 × 192 mm, voxel size 1.7 × 1.7 × 6 mm, interslice gap 4 mm.) was implemented to acquire short-axis cine-loops, covering the entire left ventricular cavity from base to apex. No contrast agents were administered. An example of a short axis image of both DSCT and MRI is provided in Fig. 1.
Visual quality assessment
Before post-processing, all DSCT datasets were visually scored for image-quality. Contrary to the quality-assessment of the DSCT dataset for coronary artery analysis, where the opacification of the coronary arteries and the ability to follow them from base to distal is essential, the quality assessment for LV functional analysis focuses more on opacifictaion of the LV cavity and the presence of “stairstep” artifacts caused by ventricular extra-systoles, breathing artifacts or segmentation errors [10].
An example of a “stairstep” artifact is shown in Fig. 2. Four categories were used: good quality (without any artifacts), minor artifacts (however, LV delineation sufficiently possible), major artifact (LV delineation not reliable) and poor image quality (no full coverage of LV or overall distortion). Image quality was scored separately by two observers, blinded for each others results. Only the DSCT datasets rated to be either of good quality and those scored to have minor artifacts were analyzed.
Post-processing
Post-processing was performed using a dedicated software package, Mass (Medis, Leiden, the Netherlands), for both DSCT and MRI data. The two dedicated post-processing tools make use of the same algorithm and visual interface. The DSCT data were reformatted to short-axis cine-loops of the LV cavity with a slice thickness of 6 mm and an interslice gap of 4 mm, matching the MRI specifications. The end-diastolic phase was defined as the phase with the greatest visually estimated luminal cavity at mid-ventricular level, and the systolic phase as the phase with the smallest luminal cavity. Readers manually traced the area of the cavity in each slice in the appropriate phases. The obtained parameters of the analysis consisted of the end-diastolic volumes (EDV) and end-systolic volumes (ESV). From these parameters stroke volume (SV) and left ventricular ejection fraction (LVEF) were calculated. Stroke volume was calculated by SV = EDV - ESV. The ejection fraction was calculated by EF = (SV/EDV) × 100%. Myocardial mass was calculated by delineating the epicardial contour as well as the endocardial contour, thereby isolating the myocardial muscle volume. By multiplying this volume by a volume-weight constant, myocardial mass was calculated. The papillary muscles were regarded as part of the ventricular cavity in all analyses. All DSCT analyses were performed by a single blinded investigator. All MRI analyses were performed by one single investigator, blinded for the DSCT data.
Data analysis
The method of Bland and Altman was used to display the average difference and limits of agreement between the reference values of MRI and the functional parameters of DSCT [11].
The results are given in the form of mean ± 2SD. A small difference to the reference is associated with high accuracy and small standard deviations are associated with high precision. Pearson’s correlation coefficient was calculated to assess the correlation between MRI and DSCT.
Results
Thirty-four consecutive patients underwent both DSCT and MRI assessment of global LV function parameters. The calculated mean effective radiation dose based on the used scan protocol is 7.3 mSv [12].
Indications for DSCT, delay to MRI and image quality of DSCT are displayed in Table 2. Interobserver-agreement for image quality of DSCT datasets was excellent and results are shown in Table 3. Twenty-nine DSCT datasets were considered to be of sufficient quality to be analyzed [good quality (n = 16) and minor artifacts (n = 13)].
Ventricular volumes
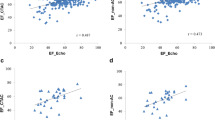
Average EDV measured by DSCT was 214.8 ml (±87.3 ml). Average EDV measured by MRI was 203.8 ml (±98.4 ml). Average ESV measured by DSCT was 94.9 ml. (±56.8 ml). Average ESV measured by MRI was 91.4 ml (±64.1 ml). The average overestimation of DSCT for EDV was 10.96 ml. (±27.4 ml.) and 3.54 ml. (±15.9 ml.) for ESV. Bland-Altman intervals for EDV and ESV were [−43.83 (10.96) 65.76] and [−28.34 (3.54) 35.42], respectively. The Bland-Altman plots for EDV and ESV are displayed in Figs. 3 and 4. The Pearson’s correlation coefficient for EDV and ESV were 0.96 and 0.97, respectively (p < 0.001 for both).
Myocardial mass
Average myocardial mass measured by DSCT was 122.1 g (±46.2 g). Compared with myocardial mass measured by MRI, there was an average overestimation of 0.9 g. Average myocardial mass measured by MRI was 121.2 g. (±46.5 g) The Bland-Altman interval for myocardial mass was [−31.0 (0.9) 32.8]. The Bland-Altman plot for myocardial mass is shown in Fig. 5. The Pearson’s correlation coefficient for myocardial mass was 0.94 (p < 0.001).
LVEF
The average LVEF measured by DSCT datasets was 57.3%-point (±8.1%-point). Compared with the average LVEF measurement by MRI of 56.9%-point (±9.0%-point), there was an average overestimation of 0.40%-point (±4.54%-point). The Bland-Altman interval for LVEF was [−8.67 (0.40) 9.48] (Fig. 6). The Pearson’s correlation coefficient for LVEF was 0.90 (p = 0.001).
Discussion
The present study shows that global LV functional parameters, calculated from DSCT datasets acquired in daily clinical practice show acceptable limits of agreement with global LV parameters obtained with MRI.
The use of MDCT for the assessment of LV functions has been investigated extensively. Studies using older generation CT showed that function assessment could be assessed, but the temporal resolution of the four- and 16-slice MDCT systems hampered the sensitivity for detection and accurate classification of regional LV wall motion abnormalities [13].
Although the temporal resolution can be improved with multi-segment reconstruction, leading to better reproducibility in a phantom study [14], it was shown that this technique did not improve results for studies in human subjects [15].
More recently introduced MDCT devices capable of achieving better temporal resolution were studied previously. Butler et al. [16] concluded that 64-slice MDCT could serve as an alternative for suboptimal echocardiography results, and Salem et al. [17] found that 64-slice MDCT enabled LV function analysis in patients with regular sinus rhythm.
A phantom study by Mahnken et al. [14] showed that reliable assessment of LV volumes was also possible at increased heart rates.
Since DSCT has only recently been introduced, there are few data on the diagnostic accuracy of this technique. A recent study using a moving heart-phantom by Mahnken et al. [18] showed a good correlation between DSCT and MRI, with no significant differences, which is in accordance to the present in-vivo study.
Only two small studies in patients are available at the present time [19, 20]. A striking difference between the two studies is the wide B-A interval in the study by Busch et al. [20] in which two different post-processing software-packages have been implemented for the MRI and DSCT on the one hand, and the narrow B-A interval in the study by Brodoefel et al. [19] in which the same post-processing tool was used for both investigations on the other hand, suggesting a possible effect of the post-processing on the results.
A number of different post-processing software packages are currently commercially available for both DSCT and MRI. Little is known regarding the accuracy and interchangeability of these post-processing tools and this needs to be elucidated before these other software packages are used in clinical practice. Since in the present study both the DSCT and the MRI data were analyzed using the identical short-axis parameters and post-processing software (Mass; Medis, Leiden, The Netherlands) the differences in volume assessment are not considered to be influenced by differences in post-processing. Since DSCT datasets cover the entire left ventricle, it is tempting to speculate that reconstruction of short-axis slices without interslice gap or even partially overlapping slices may further enhance diagnostic accuracy. However, data regarding those protocols are currently unavailable.
One of the major findings of the present study is the fact that clinical reliability of LV functional assessment can be improved dramatically if the raw DSCT dataset is checked for artifacts, influencing the short-axis cine-loops. In comparison with coronary artery analyses, when only single frames from a manually selected phase in the cardiac cycle are used, the quality analyses for LV functional analyses focus more on LV wall motion delineation. It is, therefore, important to note the fact that if coronary artery analysis is possible on a particular DSCT dataset, this does not directly imply that accurate LV functional analysis is possible as well.
The average delay between DSCT and MRI of 6.3 days in the present study can be considered a limitation. Some previous studies using other CT systems have examined patients with less delay. It is conceivable that changes in either volume status or heart rate may have influenced the intrinsic volumes of the individual patients over time, thereby influencing the direct comparison between the two imaging modalities. However, LVEF is known to be notoriously stable over time and care was taken to confirm that no significant clinical interventions or changes in medication had taken place between the two investigations in all patients.
Although MRI and DSCT both provide two- and three-dimensional anatomical information, each technique has its own specific advantages and drawbacks. DSCT is particularly useful in patients with intracorporal devices such as pacemakers and pacemakerleads. These devices cause large image artifacts on the MRI and their intra-cardiac leads may warm up and possibly even cause perforation at the tip. Although there is increasing evidence that MRI examination may be performed safely in some specific cases, pacemakers and defibrillators still remain a contraindication [21].
DSCT is a very fast technique, which allows examination of less stable patients, patients with intra-corporal devices, severely claustrophobic patients or patients who cannot lie flat on their back for the time necessary for MRI assessment.
However, DSCT requires the use of ionizing radiation. Radiation exposure is currently widely debated and many efforts have been made to reduce the effective dose in DSCT. In the present study, a retrospective triggered protocol was implemented, covering the entire RR-interval. However ECG pulsing was used to vary the tube current in order to reduce radiation exposure in the part of the cardiac cycle which is usually ignored in coronary artery patency analysis. Recently, a number of reports have been published on full prospective ECG triggering, significantly reducing the effective radiation dose, at equal image quality [22, 23].
However, it should be noted that LV function analysis is not possible using these protocols, as the maximal end-diastolic and end-systolic phase cannot be determined and may even not be covered by the scanning window.
Conclusion
Volumetric analysis renders interesting adjunctive data in DSCT performed for coronary artery evaluation. Global LV functional parameters calculated from DSCT datasets acquired in daily clinical practice correlate well with MRI and may be considered interchangeable. However, visual assessment of the image quality of the short-axis cine slices should be performed to detect any artifacts in the DSCT data which could influence accuracy.
References
Moise A, Bourassa MG, Theroux P et al (1985) Prognostic significance of progression of coronary artery disease. Am J Cardiol 55:941–946
Emond M, Mock MB, Davis KB et al (1994) Long-term survival of medically treated patients in the Coronary Artery Surgery Study (CASS) Registry. Circulation 90:2645–2657
Levy D, Garrison RJ, Savage DD et al (1990) Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 322:1561–1566
Sechtem U, Pflugfelder PW, Gould RG et al (1987) Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology 163:697–702
Rominger MB, Bachmann GF, Pabst W et al (2000) [Left ventricular heart volume determination with fast MRI in breath holding technique: how different are quantitative heart catheter, quantitative MRI and visual echocardiography?] Rofo 172:23–32
Thiele H, Paetsch I, Schnackenburg B et al (2002) Improved accuracy of quantitative assessment of left ventricular volume and ejection fraction by geometric models with steady-state free precession. J Cardiovasc Magn Reson 4:327–339
Tornqvist E, Mansson A, Larsson EM et al (2006) It’s like being in another world—patients’ lived experience of magnetic resonance imaging. J Clin Nurs 15:954–961
Schuijf JD, Bax JJ, Shaw LJ et al (2006) Meta-analysis of comparative diagnostic performance of magnetic resonance imaging and multislice computed tomography for noninvasive coronary angiography. Am Heart J 151:404–411
van der Vleuten PA, Willems TP, Gotte MJ et al (2006) Quantification of global left ventricular function: comparison of multidetector computed tomography and magnetic resonance imaging. A meta-analysis and review of the current literature. Acta Radiol 47:1049–1057
Kroft LJ, De Roos A, Geleijns J (2007) Artifacts in ECG-synchronized MDCT coronary angiography. AJR Am J Roentgenol 189:581–591
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Gerber TC, Kuzo RS, Morin RL (2005) Techniques and parameters for estimating radiation exposure and dose in cardiac computed tomography. Int J Cardiovasc Imaging 21:165–176
Fischbach R, Juergens KU, Ozgun M et al (2007) Assessment of regional left ventricular function with multidetector-row computed tomography versus magnetic resonance imaging. Eur Radiol 17:1009–1017
Mahnken AH, Hohl C, Suess C et al (2006) Influence of heart rate and temporal resolution on left-ventricular volumes in cardiac multislice spiral computed tomography: a phantom study. Invest Radiol 41:429–435
Juergens KU, Maintz D, Grude M et al (2005) Multi-detector row computed tomography of the heart: does a multi-segment reconstruction algorithm improve left ventricular volume measurements? Eur Radiol 15:111–117
Butler J, Shapiro MD, Jassal DS et al (2007) Comparison of multidetector computed tomography and two-dimensional transthoracic echocardiography for left ventricular assessment in patients with heart failure. Am J Cardiol 99:247–249
Salem R, Remy-Jardin M, Delhaye D et al (2006) Integrated cardio-thoracic imaging with ECG-gated 64-slice multidetector-row CT: initial findings in 133 patients. Eur Radiol 16:1973–1981
Mahnken AH, Bruder H, Suess C et al (2007) Dual-source computed tomography for assessing cardiac function: a phantom study. Invest Radiol 42:491–498
Brodoefel H, Kramer U, Reimann A et al (2007) Dual-source CT with improved temporal resolution in assessment of left ventricular function: a pilot study. AJR Am J Roentgenol 189:1064–1070
Busch S, Johnson TR, Wintersperger BJ et al (2008) Quantitative assessment of left ventricular function with dual-source CT in comparison to cardiac magnetic resonance imaging: initial findings. Eur Radiol 18:570–575
Roguin A, Zviman MM, Meininger GR et al (2004) Modern pacemaker and implantable cardioverter/defibrillator systems can be magnetic resonance imaging safe: in vitro and in vivo assessment of safety and function at 1.5 T. Circulation 110:475–482
Earls JP, Berman EL, Urban BA et al (2008) Prospectively gated transverse coronary CT angiography versus retrospectively gated helical technique: improved image quality and reduced radiation dose. Radiology 246:742–753
Husmann L, Valenta I, Gaemperli O et al (2008) Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 29:191–197
Conflict of interest
None declared.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van der Vleuten, P.A., de Jonge, G.J., Lubbers, D.D. et al. Evaluation of global left ventricular function assessment by dual-source computed tomography compared with MRI. Eur Radiol 19, 271–277 (2009). https://doi.org/10.1007/s00330-008-1138-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-008-1138-z