Abstract
Strong environmental seasonality is a basic feature of the Arctic system, still there are few published records of the seasonal variability of the Arctic marine biota. This study examined the year-round seasonal changes of soft bottom macro- and meiobenthic standing stocks and diversity on a station located in an Arctic fjord (Adventfjorden, Spitsbergen). The seasonality observed in benthic biota was related to the pelagic processes, primarily the seasonal fluxes of organic and inorganic particles. The highest abundance, biomass and richness of benthic fauna occurred in the spring after the phytoplankton bloom. During the summer, when a high load of glacial mineral material was transported to the fiord, the number of both meio- and macrobenthic individuals decreased remarkably. The strong inorganic sedimentation in summer was accompanied by a decline in macrobenthic species richness, but had no effects on evenness. Redundancy analysis (RDA) pointed to granulometric composition of sediments (depended on mineral sedimentation) and organic fluxes as factors best related to meio- and macrobenthic taxonomic composition, but no clear seasonal trend could be observed on the nMDS plots based on meiobenthic higher taxa or macrobenthic species abundances in the samples. This study addresses the possible effects of changes in the winter ice cover on the fjordic benthic systems because it was performed in a year with no ice cover on the fjord.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Strong seasonality is one of the basic features of Arctic ecosystems. The seasonal changes in light conditions, air temperatures, precipitation, snow and ice cover and glacier activity influence both terrestrial and marine biota (Loeng et al. 2005). Organisms dwelling in the Arctic seas must adapt to strong fluctuations in primary productivity and long periods of limited food supplies during the polar night. Despite the tremendous role of seasonality on the functioning of the system, it has received little attention. Most marine ecological studies in the Arctic have been performed in the spring or summer when the polar seas are more logistically accessible. The few published year-round marine ecological studies have focused on the pelagic biota (Anderson 1981; Węsławski et al. 1988), benthic meiofauna (Feder and Paul 1980) or selected macrobenthic invertebrate life cycles (Thorson 1936; Jewett and Feder 1977; Petersen 1978). The seasonal changes in the structures of Arctic benthic communities have not been studied.
The benthic community structure is dependent on a variety of factors that are usually interconnected, and the separation of their effects can be difficult. It is important to consider the difference between biotic and abiotic processes like recruitment and disturbance. The transport, settling and growth of larvae are the initial processes in benthic community development. They occur every year in accordance with natural life cycles. Environmental factors can limit those processes and drive the patterns of benthic distributions via post-settlement mortalities or dispersal. Low temperatures, seasonal carbon fluxes to the sea bottom and physical disturbance of sediments, including iceberg scouring and the sedimentation of glacial mineral material, are factors known to regulate the temporal and spatial variability of benthic fauna (Grebmeier and Barry 1991; Piepenburg et al. 2001; Włodarska-Kowalczuk et al. 2005).
The sedimentation rate of biogenic matter links the dynamics of benthic and pelagic communities in the Arctic region because it determines the food supply for the benthos (Węsławski et al. 1988; Wassmann et al. 1999; Piepenburg 2005). The quality and quantity of organic matter that descends to the sea floor depends on a variety of factors, including primary production, zooplankton grazing pressure, water column stratification and land runoff (Grebmeier et al. 1989). Organic matter produced pelagically can be exploited in situ to provide food for planktonic organisms, or it can be transported to the bottom, providing potential food for benthic biota (Marcus and Boero 1998). Other important determinants of benthic spatial and temporal patterns are connected with sedimentary conditions. Sediment stability and sedimentation processes appear to be more important factors controlling benthic fauna than the granulometric composition of sediments (Snelgrove and Butman 1994). Benthic biomass and diversity can be significantly decreased in the inner parts of Arctic fjords where organisms are confronted with physical disturbances produced by high turbidity, particle flux and frequent sediment resuspension and redeposition (Görlich et al. 1987; Włodarska-Kowalczuk et al. 1998, 2005).
The aim of this study was to examine the seasonal changes of the benthic fauna in relation to the seasonality of hydrological and sedimentation conditions in an Arctic subpolar fjord. The seasonal changes of both meio- and macrobenthic abundance, biomass and diversity are documented and related to processes operating in the pelagic realm during a one-year span. The seasonal changes in hydrology, flux of particles (mineral, organic, chlorophyll and phaeophytin) and faecal pellets at the same sampling site during the same time period was described in detail by Zajączkowski et al. (2010). This study reports the seasonal variability of benthic fauna in a fjord that was not covered with ice in winter time. This is, in our knowledge, the first year-round study of the benthic standing stocks and diversity performed in polar coastal waters.
Study area
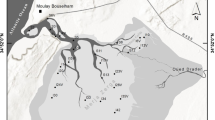
Adventfjorden is 8.3 km long and 3.4 km wide, and it is the southern arm of Isfjorden, the largest fjord of Spitsbergen. The mouths of two braided rivers, Adventelva and Longyearelva, are located in the innermost part of the fjord (Fig. 1). During the melting period, which is restricted to 122 days (Węsławski and Szymelfenig 1999), the rivers transport meltwater from glaciers situated several kilometres from the shore. In the winter, when the rivers are frozen, the supply of terrigenous material ceases.
At the head of the fjord, there is a tidal flat that is 0.7 km wide during the ebb and has a bottom inclination of less than 0.1°. The steep prodelta slope reaches an inclination of 15–19°. The depth in the central part of the fjord varies from 70 to 80 m and increases to 100 m at the fjord mouth (Fig. 1). The wide, deep entrance in Adventfjorden enables water exchange with the central part of Isfjorden due to tidal pumping and wind-driven surface currents. The tides are semidiurnal with a range of 159 cm (Zajączkowski and Włodarska-Kowalczuk 2007).
Considering the northern position of Spitsbergen, the climate of the region is relatively mild, due to the influence of the West Spitsbergen Current (WSC) transporting warm and saline Atlantic water. West Spitsbergen fjords are geographically included in the Marine Arctic, but are much warmer in terms of climatic conditions than other localities located at similar latitude (Hisdal 1985). According to Hisdal (1985), the average annual air temperature in Adventfjorden is about −6°C. The warmest months are July and August (5–6°C), and the coldest period is from January to March (−15°C). The average winter sea ice thickness ranges from about 80 cm over the central part of the fjord to more than 1 m on the tidal flat (Węsławski and Szymelfenig 1999). However, from 2005 to 2008, the fjord remained open throughout the winter (Zajączkowski et al. 2010).
Seasonal changes of hydrology and vertical particles flux at a sampling station
Seasonal changes in the hydrological and sedimentary settings are produced by the interplay of the variability in the weather conditions and the pelagic processes (Zajączkowski et al. 2010). From November to May, the water column was well mixed without distinct stratification and with relatively stable salinity values (varied between 34.2 and 34.7 PSU; Zajączkowski et al. 2010). The water temperature varied from 1.2 to 0°C during November to March and cooled down to −1.2°C in April. Warming of the water column started in May and proceeded into the summer, reaching the maximum temperature in August (7.4°C). The maximum melting period occurred during the summer months (July and August), and the surface salinity decreased to less than 5 PSU due to the increase in the freshwater supply. The reduction in the surface salinity produced water column stratification. The strongest pycnocline was formed at a depth of 2 m; however, the influence of the freshwater input was observed down to 30 m. Cooling started in October, and the stratification of the water column disappeared.
The winter flux of mineral particles was low (<10 g m−2 day−1), and suspended minerals originated from the resuspension of bottom sediments (Zajączkowski et al. 2010). The sedimentation rate of organic matter at the bottom layer reached its annual minimum in November and February (~2 g m−2 day−1). A high particulate organic carbon (POC) to nitrogen (PON) ratio in settled organic matter indicated degraded material. The flux of mineral and organic particles in the upper water column during the spring was comparable to the low winter values. However, the rate of organic material settling to the bottom showed a slight increase, and the sedimenting material was dominated by the large cells. The settling of phytoplankton reached its annual maximum in April during the bloom. The chlorophyll flux to the bottom in April was more than ten times higher than throughout the rest of the year. In May, a large part of the pigmented material that settled on the bottom was already degraded to phaeophytin. During the peak of the melting period (July and August), sedimentation of the mineral matter reached the annual maximum with more than 120 g m−2 day−1. The organic particles flux reached its maximum in August (12.4 g m−2 day−1). During the two summer months, 60% of the annual mineral matter and 53% of the annual organic matter settled at the sampling site. The increase in the POC/PON ratio in the sedimenting matter indicated decomposed organic material, and the very low chlorophyll a/phaeophytin ratio indicated heavy grazing pressure in the summer months. In this period, zooplankton development caused a significant increase in the faecal pellet flux to the bottom, which reached a maximum of 5.2 mg C m−2 day−1 in August (Zajączkowski et al. 2010).
Materials and methods
Sampling
The sampling was carried out at one sampling station, located in the inner part of Adventfjorden (Fig. 1). Samples were taken in:
-
Winter (polar night): 6th of November 2006, 6th of February 2007 and 24th of October 2007.
-
Spring: 17th of April 2007 and 22nd of May 2007.
-
Summer: 18th of July 2007 and 15th of August 2007.
The sampling location and times are identical to those outlined in Zajączkowski et al. (2010). Three Petit Ponar grabs (surface area 0.045 m2) and three sediment cores (inner diameter 2.1 cm, surface area 3.8 cm2) were taken during each sampling campaign to collect macro- and meiobenthic fauna, respectively. The sampling was performed from a small rubber boat, and it was not possible to use a grab of a standard size, i.e., 0.1 m2 van Veen grab. Włodarska-Kowalczuk et al. (2007) reported no significant differences in the macrobenthic density, biomass and species composition between samples collected in Adventfjorden with the use of a Petit Ponar grab or a van Veen grab. It should be noted that the size of a Petit Ponar sample is small and larger bodied, deeper burrowing, longer-lived species can be under-sampled with possible consequences for biomass measurements. Macrofaunal samples were sieved using a 0.5-mm sieve and fixed in 4% formaldehyde. Meiofauna was preserved in 4% formaldehyde and stained with Bengal Rose. An additional sediment core was collected for grain size, water and organic matter content analyses.
Laboratory analysis
Macrofauna was sorted, identified to the lowest possible taxonomic level and counted. The wet formalin weight of each phyla was estimated. Molluscs were weighed with shells, which is commonly conducted in benthic surveys (Holme and McIntyre 1971). It can provide some biases as the biomass estimate is not limited to the living tissue, but contains non-respiring carbonate. However, the small size of samples collected in this study lessens the probability of encountering a single large heavily calcified animals and error between biomass with and without shells is consistent across samples collected throughout the year. Meiofauna retained on a 38-μm sieve was separated from sediment by Ludox floatation and centrifugation (McIntyre and Warwick 1984). Organisms were identified to the major taxa and counted under a stereomicroscope. The volumetric method and conversion factors were used to estimate the meiobenthic biomass (Feller and Warwick 1988). The water content in the sediment was calculated as the mass lost after drying at 60°C. The organic matter content in the sediment was calculated as the mass loss after combusting dried sediments at 450°C for 24 h. Sediment granulometry was made with a Malvern Mastersizer 2000 Laser Analyser.
Data analysis
Macrofaunal density and biomass are presented as values per 1 m2. The meiobenthic density and biomass are given as values per 0.1 m2. Non-metric multidimensional scaling (nMDS) of the Bray–Curtis similarity between samples, calculated on double-root transformed data for all samples, was carried out on two data matrixes: meiobenthic major taxa and macrobenthic species abundances in the samples. The data were double-root transformed to reduce the influence of numerically dominant species. The benthic species richness and diversity indices were calculated. The species richness was expressed as the number of species per sample (macrofauna) or the number of higher taxa per sample (meiofauna). The macrobenthic species diversity was measured using the Shannon–Wiener log-based index (H′). The evenness (or equitability) of distribution of individuals among species was calculated as the Pielou index (J′). Multivariate analysis and calculation of diversity indices was performed with the use of PRIMER v. 6 software (Clarke and Warwick 1994). Constrained ordination techniques were used to explore the relationship between meio- and macrobenthic species composition and environmental properties (ter Braak and Smilauer 2002). The set of environmental variables available for the analyses included the month, inorganic vertical flux, organic vertical flux, chlorophyll a flux (data published in Zajączkowski et al. 2010), clay, silt, sand, water and organic matter content in the sediment. The clay, silt, sand and water content in the sediment were highly correlated (R = 0.8–1), so only the sand content was used in the analysis. The results of a preliminary de-trended correspondence analyses (DCA) pointed to redundancy analysis (RDA) as the most appropriate for both meio- and macrobenthic data. The forward selection of environmental variables was used to quantify and rank the importance of variables in determining the species composition. The effects of environmental variables were tested, with Monte Carlo permutation tests that used 499 unrestricted permutations. The analyses were carried out using CANOCO v. 4.5 software (ter Braak and Smilauer 2002).
Results
Sediment
The grain size composition, water content and organic matter content in the surface sediment varied seasonally in Adventfjorden (Fig. 2). In the winter, the sand content increased to more than 20%, and the clay almost disappeared. In this period, the pore water and organic matter content decreased to the annual minima of 40 and 1.2%, respectively. However, in November the organic fraction of sediment reached the summer level (3.9%). In two spring months (April and May), the organic matter content reached its annual maximum (6.7%). In May, the finest grains fraction reappeared in the surface sediment (10%) and increased up to 15% during the melting period. The highest pore water content was also observed in August (56%). In the summer, the organic matter content in the surface sediments decreased to 4% and was lower than in the spring months even though the sedimentation of organic matter reached its annual maximum in August (Zajączkowski et al. 2010) (Fig. 2).
Meiofauna
Meiofaunal densities ranged from 942 to 11,006 ind. per 0.1 m2. The highest densities were observed in the spring (April and May) and in the winter (November and October). The lowest values were noted in February and during the summer melting season (July and August) (Fig. 3). Ten major meiofaunal taxa were recorded in Adventfjorden. The most diverse meiofaunal samples were collected in April and May (10 taxa), and the lowest diversity was observed in November (5 taxa) (Fig. 4). Regardless of the season, more than 90% of all individuals were represented by Nematoda. Harpacticoida and Polychaeta larvae were other permanent components of the meiofauna. Ostracods, cnidarians, copepods nauplii, turbellarians and oligochaetes were also present in the meiofaunal size fraction; however, their occurrence and densities were variable throughout the studied time (Table 1 and Fig. 3). The biomass of meiofauna varied from 2.7 to 17.1 μg ww 0.1 m−2. In most samples, it was dominated by Nematoda and Polychaeta (Fig. 5). No clear seasonal trend in the meiobenthic higher taxa composition in Adventfjorden could be detected with the use of nMDS analysis (Fig. 6). RDA indicated that the vertical flux of chlorophyll a, the month and sand content in the sediment had significant influences on the meiofaunal species composition and explained 21, 13 and 19% of the total variability, respectively. The complete model explained 52.6% of the total variability (Table 3).
Macrobenthos
Forty-two macrobenthic species were identified, mostly Polychaeta (52%), Mollusca (33%) and Crustacea (7%). The macrofauna was dominated by polychaetes Chaetozone spp. (10 to 35% in July and November, respectively). Eteone spitsbergensis, Cirratulus sp., Heteromastus filiformis and Cossura longocirrata also occurred in large numbers. The bivalves Liocyma fluctuosa and Axinopsida orbiculata as well as the gastropods Cylichna alba and Cylichna occulta were the most numerous molluscs (Table 2). The macrobenthic total densities varied seasonally with the highest values in April and the lowest in July. Throughout the year, polychaetes were the most numerous, and molluscs were dominant only in July (Fig. 7a). The macrobenthic biomass varied from 11 (November) to 130 (May) g ww per m−2 and was dominated by molluscs (Fig. 7b).
The species-rich samples were collected from November to May, and the highest mean number of macrobenthic species per sample (19) was recorded in April (Fig. 8). The Shannon–Wiener index (H′) varied from 1.6 (July) to 2.2 (February). High values of this index were recorded from November to May. The Pielou index (J′) was the lowest in May (0.79) and the highest in October (0.87) (Fig. 8).
No clear seasonal trend in the macrobenthic composition in Adventfjorden could be detected on the nMDS plot (Fig. 9). Three of the variables tested with the Monte Carlo permutation test had a significant influence on the macrobenthic species composition: inorganic vertical flux, organic vertical flux and sand content in the sediment. The model explained 44.6% of the total variability. The inorganic flux, organic flux and sand content explained 23, 10 and 12%, respectively (Table 3).
Discussion
The benthic diversity, abundance and biomass showed clear seasonal patterns with the highest values recorded in the spring and the lowest in the summer and winter. Seasonality was evident for both meio- and macrofauna. Vertical fluxes of organic and mineral matter controlled both the food supply and substrate conditions and seemed to be the driving factors for the observed seasonal changes in the diversity and standing stocks of benthic biota. The results of the RDA indicate the high impact of both organic and inorganic fluxes on benthos, suggesting that both physical disturbances, caused by inorganic matter supplied with glacial meltwaters, and the food supply can drive changes in the benthic structure.
Winter abundance and biomass
In the winter months, the macrobenthic biomass was much lower than that observed in the spring or summer. The density and number of taxa were also slightly lower. In November of 2006, the meiofaunal abundance and biomass were relatively high. In February of 2007, the abundance was slightly higher than the annual minimum, and the biomass was low. In October of 2007, increases in the meio- and macrofaunal densities and biomass were observed. During the winter the rivers were frozen, and the terrestrial material supply was at an absolute minimum, resulting in relatively undisturbed substrate conditions for the benthic fauna when compared to the summer conditions. Winter is also a period when food supply is limited—primary production during polar night is stunted. Despite this fact, the particulate organic matter (POM) flux in the near-bottom layer was comparable to the values observed in May (Zajączkowski et al. 2010). The main food source during this period was probably provided by resuspended particles in the near-bottom water layers and/or organic matter deposited in sediments. Atypically high meiofaunal abundance and diversity in November 2006 probably resulted mainly from the reduction of the disturbance level due to a lower sedimentation rate, compared to summer months.
Spring abundance and biomass
The highest benthic abundance and diversity was documented in April, immediately after the phytoplankton bloom when recently produced organic matter became available (Fig. 10). The spring phytoplankton bloom is responsible for most of the total annual organic carbon supply to Arctic fjord marine ecosystems (Wiktor 1999). In Arctic ecosystems, the timing and rates of primary production is related to the presence and thickness of the sea ice cover. The most important effect of sea ice observed in West Greenland is the shortening of the productive period. Sea ice, covered with snow, reflects almost all the sunlight, and the phytoplankton bloom can occur only after the ice disappears (Petersen 1977). Petersen (1964) noted that the peak of primary production in the Disko Bay (West Greenland) took place in May–June, just after the ice breakup. A similar situation was observed in Adventfjorden in 1995 when the phytoplankton bloom occurred in May after the ice cover was reduced from 65 to 15 cm, and the euphotic zone extended to 15 m (Wiktor 1999). In winter 2006/2007, water in Adventfjorden was not sufficiently cooled to form an ice cover. The lack of sea ice advanced the primary production and accelerated the bloom. The main phytoplankton biomass appeared in April. High numbers of zooplankton occurred as a response to the phytoplankton bloom, exerting considerable grazing pressure on the particulate matter and phytoplankton population. Furthermore, increased grazing did not seem to be followed by an increase in the faecal pellet vertical flux. The Adventfjorden zooplankton in the spring consisted mostly of Cirripedia nauplii, which produce small faecal pellets that probably remain in suspension (Zajączkowski et al. 2010). Glud et al. (2007) predicted that primary production in Young Sound, Greenland would benefit from the thinning of sea ice and increase import of nutrients, resulting from the increased exchange between the fjord and the Greenland Sea as a result of climate change. This would lead to an increase in primary production and organic matter sedimentation and thus to an intensification of the decomposition processes at the sea bottom. The higher sedimentation would stimulate the development of benthic communities, but intensive bacterial mineralisation would reduce oxygen availability in the sediment. In Adventfjorden enhanced primary productivity did not lead to increased sedimentation. Heavy grazing pressure combined with a small number of faecal pellets resulted in a lower flux of organic matter, compared with previous ice-covered years (Zajączkowski et al. 2010). From a benthic perspective, the lower organic flux means a reduction of the food base, and this can result in a reduction of the standing stocks of benthic communities.
The highest numbers of juveniles were observed in the spring months (April and May). The effect of recruitment, apart from fluxes of suspended matter, may be another factor that contributes to the seasonal changes of benthic standing stocks. In an Arctic environment, phytoplankton outburst seems to be an inducing factor for the spawning of many invertebrate species (Petersen 1977). Petersen (1978) observed the highest feeding and breeding activity of mollusks in the Disko Bugt (West Greenland) during the spring bloom, when temperature was still low. To match in time with the spring bloom, organisms should release larvae just before or during the productive period (Węsławski et al. 1991); thus, most of the larvae of benthic animals are present in coastal plankton from April to early June (Węsławski et al. 1988). In the boreal and temperate areas, development of invertebrates is directly influenced by the environmental temperature (Todd and Doyle 1981). Most species reproduce in spring (Thorson 1946), when temperature is rising, but some invertebrates are able to reproduce also in winter. Buhl-Jensen and Fossa (1991) observed in Gullmarfjord (western Sweden) a general tendency for neritic mysids to be most abundant in spring and winter. Mysid Erythrops erythrophthalma seemed to have reproductive maximum in winter, but juveniles were observed year-round. Maximum reproduction in spring as well as second maximum in autumn has been observed for many boreal shallow-water amphipods (Sainte-Marie and Brunel 1983; Carrasco and Arcos 1984; Costello and Myers 1989).
Summer abundance and biomass
In summer months, the input of terrigenous material transported by the rivers produced high particle flux, which affected both the benthic food availability and substrate conditions. The organic matter content in surface sediments was lower in the summer than in the spring (Zajączkowski et al. 2010). The decrease in the organic matter concentration in sediments resulted from a “dilution” of the organic matter in the mass of inorganic particles (Görlich et al. 1987). The high supply of terrigenous material also produced a signal in the granulometric composition of sediments. The silt and clay fractions made up 95% of the sediment in July and August. Also, the water content in the sediment increased substantially from 47% in May to about 57% in July and August. Large loads of fine deposits lead to the formation of a layer of unconsolidated, easily eroded sediments that are frequently resuspended and redeposited (Syvitski and Shaw 1995; Zajączkowski and Włodarska-Kowalczuk 2007).
Physical disturbances caused by the large terrigenous inflow in the summer led to a reduction in the numbers and biomass of both meio- and macrofauna in Adventfjorden sediments (Fig. 10). In July and August, the meiobenthic density and biomass was at a minimal level. Only in July the biomass of meiofauna rose, as a result of the presence of a few large individuals of Polychaeta. The abundance and biomass of macrobenthos also decreased during the summer. The strong seasonal effects of mineral sedimentation on Adventfjorden benthic standing stocks correspond well with studies reporting the spatial patterns related to the gradients of sediment disturbance. Włodarska-Kowalczuk et al. (2007) studied meio- and macrobenthic characteristics in three zones of the Adventfjorden estuary that differed significantly with relation to mineral material sedimentation and sediment stability. In the unstable sediments of the prodelta slope where high rates of inorganic sedimentation and frequent gravity-driven sediment flows occur, both meio- and macrobenthic densities and biomass were much lower than in the stable sediments of the central basin. A similar situation was observed in Kongsfjorden (a fjord off west Spitsbergen) where the macrobenthic biomass decreased with increasing proximity to the glacier (Włodarska-Kowalczuk et al. 2005). Newell et al. (1998) showed that dredging in coastal waters results in a decrease in macrobenthic standing stocks with a 40–70% reduction in the number of individuals and a 60–90% reduction in the biomass. Blanchard and Feder (2003) recorded a signal for biomass decline in a macrofaunal community disturbed by dredging in Alaskan coastal waters. They showed that benthic fauna was able to return to pre-disturbance biomass, although re-adjustment could be greater than 5 years in some cases.
The seasonal meiofaunal abundance reduction in July and August and low biomass in August contradict previous reports that indicated high resistance of meiofauna to sediment disturbance. An investigation of a benthic community in Bermuda performed by Warwick et al. (1990) showed that meio- and macrobenthos were affected differently by environmental disturbances. The densities of macrobenthos decreased significantly, while meiofauna was slightly impacted. Sherman and Coull (1980) found that despite a significant drop in meiobenthic numerical abundance after a disturbance, 12 h was sufficient for the major groups to return to pre-disturbance levels. This process could be delayed by frequently repeated disturbances combined with a low number of juveniles (Austen and Widdicombe 2006). Somerfield et al. (1995) showed that mechanical sediment disturbances connected with sediment resuspension and redeposition affect both macrofauna and meiofauna. Jewett and Feder (1977) wrote that the seasonal pattern for the abundance of the harpacticoid Harpacticus uniremis in Alaska reflects its reproductive cycle and is characterised by the sequential occurrence of successive life stages. Seasonal fluctuations in the density and biomass of meiofauna in Adventfjorden are probably the result of the interaction of environmental factors (i.e., disturbances and food supply as discussed earlier) and natural life cycles (natural mortality of adult individuals in the winter: February and October).
Seasonal changes in diversity and species composition
Different responses to summer disturbance pressures were noted when different aspects of diversity, such as species richness and evenness, were examined. A decline in species richness was not accompanied by a decrease in evenness, expressed by the Pielou index. Species diversity (measured by the Shannon–Wienner index), which combines both species richness and evenness, decreased as a result of the decline in the species richness. During the study performed along the spatial gradient of sediment disturbances in Adventfjorden, the same trend was observed—a significant decrease in species richness and no effect on evenness in the disturbed localities (Włodarska-Kowalczuk et al. 2007). Magurran (2004) also showed that the disturbance is not always accompanied by a decline in evenness and stated that species richness measures are better indicators of a perturbation than evenness or species diversity metrics.
No clear seasonal patterns were recorded when the taxonomic composition of the fauna was analysed, either for the macrofauna examined at the species level or for the meiofaunal higher taxonomic groups. The lack of clear seasonal trends in the nMDS plot could result from the low number of replicates collected and the large variability among replicates, exceeding the variability among the months (Fig. 9). The natural patchiness in the distribution of benthic organisms and the high frequency of physical disturbances may result in high variability between replicates that can mask the seasonal trends. This effect is often observed in sampling programs based on low number of replicates (Sibert 1979).
In the multivariate analyses, only August seems to be different both for meio- and macrofauna. The sedimentation of organic and mineral material proceeded from April to June, respectively. The sampling point was located on a steep slope where sedimenting material accumulated until the critical threshold when gravity generated a flow of sediment or a mixture of sediment and fluid. Long-lasting sedimentation processes make gravity flow more probable (Zajączkowski and Włodarska-Kowalczuk 2007), and the strong decline of the fauna in August could result from an episodic catastrophic event at the sampling station, e.g., the mass flow of sediments on the slope.
The taxonomic composition of the macrofauna was similar to that recorded in previous summer surveys of the Adventfjorden sediments (Holte et al. 1996; Węsławski and Szymelfenig 1999; Włodarska-Kowalczuk et al. 2007). In 2006–2007 (present study), the polychaetes Chaetozone spp., Heteromastus filiformis and C. longocirrata were the most numerous, while Włodarska-Kowalczuk et al. (2007) listed Capitella capitata, Chaetozone setosa and C. longocirrata as the dominant species on the delta slope in 2001–2002. C. capitata is a common coloniser of defaunated sediments and is very resistant to sediment instabilities (McCall 1977; Blanchard and Feder 2003). Ch. setosa and C. longocirrata are common dominants in Spitsbergen glacial bays and are resistant to high sedimentation (Włodarska-Kowalczuk et al. 1998). Communities living in fine mobile sediments are characterised by large populations of species that are well adapted to rapid recolonisation of disturbed sediments (Newell et al. 1998). Włodarska-Kowalczuk and Pearson (2004) noted that in glacial bays, known as areas with high levels of physical sediment disturbances, the fauna was dominated by small-bodied deposit-feeding species and characterised by low biomass and low diversity.
More than 90% of the meiofauna belonged to Nematoda throughout all seasons. The lowest abundances of Harpacticoid copepods were observed in May, July and August, months when sediments were disturbed by inorganic sedimentation. Experimental studies showed that nematodes migrate deep through the sediment and thereby can avoid negative effects of high sedimentation and burial (Schratzberger et al. 2004). Harpacticoida occupy the surface layer of sediment that is unstable and can be easily removed by gravity flows. Jewett and Feder (1977) observed that the harpacticoid copepod H. uniremis did not perform downward migration in sediments. Probably, harpacticoids can recolonise disturbed areas only by active or passive migration in near-bottom water (Chandler and Fleeger 1983).
Conclusions
This study shows that the seasonal dynamics of the benthic communities in an Arctic fjord are driven by pelagic–benthic coupling processes mediated by physical processes. The spring flux of organic matter connected with the plankton bloom, the summer flux of mineral particles and the peak of glaciofluvial inflows determine the levels and diversity of benthic standing stocks.
The lack of a winter ice cover could have a tremendous effect on the seasonal patterns in Adventfjorden. Petersen (1984) stated that in the Arctic regions, the benthic energy flow is greater than the pelagic one, which is opposite to tropical regions where the pelagic flow is more important. The disappearance of the sea ice cover, as observed during this study, enhanced the primary productivity but did not lead to an increase in sedimentation due to high grazing pressure and the small number of faecal pellets (Zajączkowski et al. 2010). The reduction in the organic matter flux to the bottom may cause a shift in the Arctic food webs from mostly benthic to mostly pelagic as was documented in the Arctic shelves (Grebmeier et al. 2006). Further research is needed to understand how these pelagic processes, which are triggered by changes in ice conditions, can alter the function of the fjordic benthic systems.
References
Anderson OGN (1981) The annual cycle of phytoplankton, primary productivity and hydrology in the Disko Bugt area, West Greenland. Medd om Grønl Bioscience 6:1–64
Austen MC, Widdicombe S (2006) Comparison of the response of meio- and macrobenthos to disturbance and organic enrichment. J Exp Mar Biol Ecol 330:96–104
Blanchard AL, Feder HM (2003) Adjustment of benthic fauna following sediment disposal at a site with multiple stressors in Port Valdez, Alaska. Mar Pollut Bull 46:1590–1599
Buhl-Jensen L, Fossa JH (1991) Hyperbenthic crustacean fauna of the Gullmarfjord area (western Sweden): species richness, seasonal variation and long-term changes. Mar Biol 109:245–258
Carrasco FD, Arcos DF (1984) Life history and production of a cold-temperate population of the sublittoral amphipod Ampelisca araucana. Mar Ecol Prog Ser 14:245–252
Chandler TG, Fleeger JW (1983) Meiofaunal colonization of azoic estuarine sediment in Louisiana: mechanisms of dispersal. J Exp Mar Biol Ecol 69:175–188
Clarke KR, Warwick RM (1994) Changes in marine communities: an approach to statistical analysis and interpretation. Natural Environment Research Council, Plymouth Marine Laboratory, Plymouth
Costello MJ, Myers AA (1989) Breeding periodicity and sex ratios in epifaunal marine Amphipoda in Lough Hyne, Ireland. Estuar Coastal Shelf Sci 29:409–419
Feder HM, Paul AJ (1980) Seasonal trends in meiofaunal abundance on two beaches in Port Valdez, Alaska. Syesis 13:27–36
Feller RJ, Warwick RM (1988) Energetics. In: Higgins RP, Thiel H (eds) Introduction to the Study of Meiofauna. Smithsonian Institution Press, Washington, DC, pp 181–196
Glud RN, Rysgaard S, Kühl M, Hansen JW (2007) The sea ice in Young Sund: Implications for carbon cycling. In: Rysgaard S, Glud RN (eds) Carbon cycling in Arctic marine ecosystems: Case study Young Sund. Meddr. Grønland, Bioscience 58: 62–85
Görlich K, Węsławski JM, Zajączkowski M (1987) Suspension settling effect on macrobenthos biomass distribution in the Hornsund fjord, Spitsbergen. Polar Res 5:175–192
Grebmeier JM, Barry JP (1991) The influence of oceanographic processes on pelagic-benthic coupling in polar regions: a benthic perspective. J Mar Syst 2:495–518
Grebmeier JM, McRoy CP, Feder HM (1989) Pelagic benthic coupling on the shelf of the northern Bering and Chukchi seas. I. Food supply source and benthic biomass. Mar Ecol Prog Ser 48:57–67
Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper LW, Frey KE, Helle JH, McLaughin FA, McNutt SL (2006) A major ecosystem shift in the northern Bering Sea. Science 311:1461–1464
Hisdal V (1985) Geography of Svalbard. Norsk Polarinstitutt, Oslo
Holte B, Dahle S, Gulliksen B, Naes K (1996) Some macrofaunal effects of local pollution and glacier-induced sedimentation with indicative chemical analyses in the sediments of two Arctic fjords. Polar Biol 16:549–557
Jewett SC, Feder HM (1977) Biology of the harpacticoid copepod Harpacticus uniremis Kröyer on Dayville Flats, Port Valdez, Alaska. Ophelia 16(1):111–129
Loeng H et al. (2005) Marine systems. In: Arctic climate impact assessment. Cambridge University Press
Magurran AE (2004) Measuring biological diversity. Blackwell Publishing, Malden
Marcus NH, Boero F (1998) Minireviev: the importance of benthic-pelagic coupling and the forgotten role of life cycles in coastal aquatic systems. Limnol Oceanogr 43:763–768
McCall PL (1977) Community patterns and adaptive strategies of the infaunal benthos of Long Island Sound. J Mar Res 35:221–263
McIntyre AD, Warwick RM (1984) Meiofauna techniques. In: Holme NA, McIntyre AD (eds) Methods for the study of marine benthos. Blackwell, Oxford, pp 217–244
Newell RC, Seiderer LJ, Hitchcock DR (1998) The impact of dredging works in coastal waters: a review of the sensitivity to disturbance and subsequent recovery of biological resources on the sea bed. Oceanogr Mar Biol Annu Rev 36:127–178
Petersen GH (1964) The hydrography, primary production, bathymethry and “Tagsaq” of Disko Bugt, West Greenland. Meddr Grønland
Petersen GH (1977) Biological effects of sea-ice and icebergs in Greenland. In: Dunbar MJ (ed) Polar oceans. Arctic Institute of North America, Calgary, Alberta, pp 319–329
Petersen GH (1978) Life cycles and population dynamics of marine benthic bivalves from the Disko Bugt area of West Greenland. Ophelia 17(1):95–120
Petersen GH (1984) Energy flow in comparable aquatic ecosystems from different climatic zones. Rapp P-v Reun Cons int Explor Mer 183:119–125
Piepenburg D (2005) Recent research on Arctic benthos: common notions need to be revised. Polar Biol 28:733–755
Piepenburg D, Brandt A, von Juterzenka K, Mayer M, Schnack K, Seiler D, Witte U, Spindler M (2001) Patterns and determinants of the distribution and structure of benthic faunal assemblages in the northern North Atlantic. In: Schäfer P, Riztrau W, Schlüter M, Thiede J (eds) The northern North Atlantic: a changing environment. Springer, Berlin, pp 179–198
ACIA Arctic Climate Impact Assessment Scientific Report (2005) Cambridge University Press
Sainte-Marie B, Brunel P (1983) Differences in life history and success between suprabenthic shelf populations of Arrhis phyllonyx (Amphipoda, Gammaridea) in two ecosystems of the Gulf of St. Lawrence. J Crustacean Biol 3:45–69
Schratzberger M, Whomersley P, Warr K, Bolam SG, Rees HL (2004) Colonisation of various types of sediment by estuarine nematodes via lateral infaunal migration: a laboratory study. Mar Biol 145:69–78
Sherman KM, Coull BC (1980) The response of meiofauna to sediment disturbance. J Exp Mar Biol Ecol 46:59–71
Sibert JR (1979) Detritus and juvenile salmon production in the Nanaimo Estuary: II Meiofauna available as food to juvenile chum salmon (Oncorhynchus keta). J Fish Res Board Can 36:497–503
Snelgrove PVR, Butman CA (1994) Animal-sediment relationships revisited: cause versus effect. Oceanography Mar Biol Annu Rev 32:111–117
Somerfield PJ, Rees HL, Warwick RM (1995) Interrelationships in community structure between shallow-water marine meiofauna and macrofauna in relation to dredgings disposal. Mar Ecol Prog Ser 127:103–112
Syvitski JMP, Shaw J (1995) Sedimentology and geomorphology of fjords. In: Perillo GME (ed) Geomorphology and Sedimentology of estuaries. Developments in sedimentology 53. Elsevier Sci B.V, Amsterdam, pp 113–178
ter Braak CJT, Smilauer P (2002) CANOCO reference manual and CanoDraw for Windows, user’s guide. Biometris, Wageningen and Ceske Budejovice
Thorson G (1936) The larval development, growth and metabolism of arctic marine bottom invertebrates compared with those from other seas. Medd om Grønl 100(6):1–155
Thorson G (1946) Reproduction and larval development of Danish marine bottom invertebrates, with special reference to the planktonic larvae in the Sound (Øresund). Meddr Kommn Danm Fisk-og Havunders (Ser Plankton) 4:1–523
Todd CD, Doyle RW (1981) Reproductive strategies of marine benthic invertebrates: a settlement-timing hypothesis. Mar Ecol Prog Ser 4:75–83
Warwick RM, Platt HM, Clarke KR, Agard J, Gobin J (1990) Analyses of macrobenthic and meiobenthic community structure in relation to pollution and disturbance in Hamilton Harbor, Bermuda. J Exp Mar Biol Ecol 138:119–142
Wassmann P, Hansen L, Andreassen IJ, Wexels Riser C, Urban-Rich J (1999) Distribution and sedimentation of faecal pellets on the Nordvestbanken shelf, northern Norway, in 1994. Sarsia 84:239–252
Węsławski JM, Szymelfenig M (1999) Community composition of tidal flats on Spitsbergen: consequence of disturbance? In: Gray JS et al. (eds) Biogeochemical cycling and sediment ecology, Kluwer Academic Publishers, pp 185–193
Węsławski JM, Zajączkowski M, Kwaśniewski S, Jezierski J, Moskal W (1988) Seasonality of an Arctic fjord ecosystem: Hornsund, Spitsbergen. Polar Res 6:185–189
Węsławski JM, Kwaśniewski S, Wiktor J (1991) Winter in a Svalbard Fiord ecosystem. Arctic 44(2):115–123
Wiktor J (1999) Early spring microplankton development under fast ice covered fjords of Svalbard, Arctic. Oceanol 41:51–72
Włodarska-Kowalczuk M, Pearson TH (2004) Soft-bottom macrobenthic faunal associations and factors affecting species distributions in an Arctic glacial fjord (Kongsfjord, Spitsbergen). Polar Biol 27:155–167
Włodarska-Kowalczuk M, Węsławski JM, Kotwicki L (1998) Spitsbergen glacial bays macrobenthos–a comparative study. Polar Biol 20:66–73
Włodarska-Kowalczuk M, Pearson TH, Kendall MA (2005) Benthic response to chronic natural physical disturbance by glacial sedimentation in an Arctic fjord. Mar Ecol Prog Ser 303:31–41
Włodarska-Kowalczuk M, Szymelfenig M, Zajączkowski M (2007) Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). II: Meio- and macrobenthic fauna. Estuar Coast Shelf Sci 74:274–284
Zajączkowski M, Włodarska-Kowalczuk M (2007) Dynamic sedimentary environments of an Arctic glacier-fed river estuary (Adventfjorden, Svalbard). I: flux, deposition, and sediment dynamics. Estuar Coast Shelf Sci 74:285–296
Zajączkowski M, Nygård H, Hegseth EN, Berge J (2010) Vertical flux of particulate matter in an Arctic fjord: the case of lack of the sea-ice cover, Adventfjorden 2006–2007. Polar Biol 33:223–239
Acknowledgments
This manuscript benefited from constructive reviews by Arny L. Blanchard and two anonymous reviewers. Jan Marcin Węsławski is gratefully acknowledged for helpful comments on the manuscript. Lech Kotwicki is thanked for help with the meiofaunal analysis. Adrian Zwolicki is thanked for help with the statistical analysis. The language has been improved thanks to the Editors from the American Journal Experts.
The publication was financially supported by the University Centre in Svalbard and the Polish Ministry of Scientific Research and Information Technology (328/N-IPY/2008 and N N304 156937).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Pawłowska, J., Włodarska-Kowalczuk, M., Zajączkowski, M. et al. Seasonal variability of meio- and macrobenthic standing stocks and diversity in an Arctic fjord (Adventfjorden, Spitsbergen). Polar Biol 34, 833–845 (2011). https://doi.org/10.1007/s00300-010-0940-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-010-0940-7