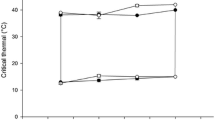
Abstract
Antarctic marine organisms are considered to have extremely limited ability to respond to environmental temperature change. However, here we show that the Antarctic notothenioid fish Pagothenia borchgrevinki is an exception to this theory. P. borchgrevinki was able to acclimate its resting metabolic rate and resting ventilation frequency after a 5°C rise in temperature. Acute exposure to 4°C resulted in an elevation in metabolic rate (57.8 ± 4.79 mg O2 kg−1 h−1) and resting ventilation rate (40.38 ± 1.61 breaths min−1) compared with fish at −1°C (metabolic rate 34.45 ± 3.12 mg O2 kg−1 h−1; ventilation rate 29.88 ± 3.72 breaths min−1). However, after a 1-month acclimation period, there was no significant difference in the metabolic rate (cold fish 29.52 ± 3.01; warm fish 31.13 ± 2.30 mg O2 kg−1 h−1), or the resting ventilation rate (cold fish 28.75 ± 0.98; warm fish 34.25 ± 2.28 breaths min−1) of cold and warm acclimated fish. Acclimation changes to the rate of oxygen consumption following exhaustive exercise were complex. The pattern of oxygen consumption during recovery from exhaustive exercise was not significantly different in either cold or warm acclimated fish.




Similar content being viewed by others
References
Alsop DH, Wood CM (1997) The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J Exp Biol 200:2337–2346
Barrionuevo WR, Fernandas MN (1998) Time-course of respiratory metabolic adjustments of a South American fish, Prochilodus scrofa exposed to low and high temperatures. J App Ichthy 14(1–2):37–41
Blazka P, Volf M, Cepela M (1960) A new type of respirometer for the determination of the metabolism of fish in an active state. Physiol Bohemoslov 9:553–560
Bouchard P, Guderley H (2003) Time course of the response of mitochondria from oxidative muscle during thermal acclimation of rainbow trout Oncorhynchus mykiss. J Exp Biol 206:3455–3465
Boyce SJ, Clarke A (1997) Effect of body size and ration on specific dynamic action in the Antarctic plunderfish Harpagifer antarcticus Nybelin 1947. Physiol Zool 70:679–690
Boyce SJ, Murray AWA, Peck LS (2000) Digestion rate, gut passage time and absorption efficiency in the Antarctic spiny plunderfish. J Fish Biol 57:908–929
Cech JJ (1990) Respirometry. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Maryland, pp 335–362
Clarke A (1991) What is cold adaptation and how should we measure it? Am Zool 31:81–92
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J An Ecol 68(5):893–905
Clarke A, Fraser KPP (2004) Why does metabolism scale with temperature? Funct Ecol 18:243–251
Davison W, Forster ME, Franklin CE, Taylor HH (1988) Recovery from exhausting exercise in an Antarctic fish, Pagothenia borchgrevinki. Polar Biol 8:167–171
Davison W, Franklin CE, Carey PW (1990) Oxygen uptake in the Antarctic teleost Pagothenia borchgrevinki. Limitations imposed by X-cell gill disease. Fish Physiol Biochem 8:69–78
De Vries AL, Eastman JT (1981) Physiology and ecology of notothenioid fishes of the Ross Sea. J Royal Soc NZ 11(4):329–340
Forster ME, Franklin CE, Taylor HH, Davison W (1987) The aerobic scope of an Antarctic fish Pagothenia borchgrevinki and its significance for metabolic cold adaptation. Polar Biol 8:155–159
Franklin CE, Johnston IA, Batty RS, Yin MC (1996) Metabolic recovery in herring larvae following strenuous activity. J Fish Biol 48:207–216
Fry FEJ (1947) Effects of the environment on animal activity. Publ Ont Fish Lab 55:1–62
Hardewig I, Pörtner HO, Van Dijk P (2004) How does he cold stenothermal gadoid Lota lota survive high water temperatures during summer? J Comp Physiol 74B(2):149–156
Huey RB, Hertz PE (1984) Is a jack-of-all-temperatures a master of none? Evolution 38(2):441–444
Jain KE, Farrell AP (2003) Influence of seasonal temperature on the repeat swimming performance of rainbow trout Oncorhynchus mykiss. J Exp Biol 206:3569–3579
Jensen FB, Nikinmaa M, Weber RE (1993) Environmental perturbations of oxygen transport in teleost fishes: causes, consequences and compensations. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman & Hall, London, pp 161–179
Jobling M (1993) Bioenergetics: feed intake and energy partitioning. In: Rankin JC, Jensen FB (eds) Fish ecophysiology. Chapman & Hall, London, pp 1–44
Johnston IA, Dunn J (1987) Temperature acclimation and metabolism in ectotherms with particular reference to teleost fish. In: Bowler K, Fuller BJ (eds) Temperature and animal cells. Symposia of the society for experimental biology, vol 41. The Company of Biologists, Cambridge, pp 67–93
Johnston IA, Battram J (1993) Feeding energetics and metabolism in demersal fish species from Antarctic, temperate and tropical environments. Mar Biol 115:7–14
Johnston IA, Temple GK (2002) Thermal plasticity of skeletal muscle phenotype in ectothermic vertebrates and its significance for locomotory behaviour. J Exp Biol 205:2305–2322
Johnston IA, Clarke A, Ward P (1991) Temperature and metabolic rate in sedentary fish from the Antarctic, North Sea and Indo-West Pacific Ocean. Mar Biol 109:191–195
Lowe CJ (2004) The effect of acute and chronic elevation of temperature on aspects of the physiology of Antarctic Nototheniid fishes. PhD Thesis. Zoology, Christchurch
Lowe CJ, Davison W (2006) Thermal sensitivity of scope for activity in Pagothenia borchgrevinki, a cryopelagic Antarctic nototheniid fish. Polar Biol 29:971–977
MacNutt MJ, Hinch SG, Farrell AP, Topp S (2004) The effect of temperature and acclimation period on repeat swimming performance in cutthroat trout. J Fish Biol 65:342–353
Manush SM, Pal AK, Chatterjee N, das T, Mukherjee SC (2004) Thermal tolerance and oxygen consumption of Macrobrachium rosenbergii acclimated to three temperatures. J Therm Biol 29:15–19
McKenzie DJ, Semini G, Piraccini G, Bronzi P, Bolis CL (1996) Effects of diet on responses to exhaustive exercise in Nile Tilapia (Oreochromis nilotica) acclimated to three different temperatures. Comp Biochem Physiol 114(A):43–50
Morris DJ, North AW (1984) Oxygen consumption of five species of fish from South Georgia. J Exp Mar Biol Ecol 78:75–86
Newell RC (1973) Environmental factors affecting the acclimatory responses of ectotherms. In: Wieser W (ed) Effects of temperature on ectothermic animals. Springer, Paris, pp 151–164
Peck LS (2002) Ecophysiology of Antarctic marine ectotherms: limits to life. Polar Biol 25:31–40
Peck LS, Webb KE, Bailey DM (2004) Extreme sensitivity of biological function to temperature in Antarctic marine species. Funct Ecol 18:625–630
Perry SF, Wood CM (1989) Control and coordination of gas transfer in fishes. Can J Zool 67:2961–2970
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Pörtner HO, van Dijk PLM, Hardewig, I, Sommer A (2000) Levels of metabolic cold adaptation: tradeoffs in eurythermal and stenothermal ectotherms. In: Davison W, Howard-Williams C, Broady P (eds) Antarctic ecosystems: models for wider ecological understanding, pp 109–122
Precht H, Christophersen J, Hensel H, Larder W (1973) Temperature and life. Springer, Berlin, pp 514
Saint-Paul U, Hubold G, Ekau W (1988) Acclimation effects on routine oxygen consumption of the Antarctic fish Pogonophryne scotti (Artedidraconidae). Polar Biol 9:125–128
Seebacher F, Davison W, Lowe CJ, Franklin CE (2005) A falsification of the thermal specialisation paradigm: compensation for elevated temperatures in Antarctic fishes. Biol Lett 1:151–154
Taylor EW, Egginton S, Taylor SE, Butler PJ (1997) Factors which may limit swimming performance at different temperatures. In: Wood CM, McDonald DG (eds) Global warming: implications for freshwater and marine fish. Society for experimental biology seminar, Series 61. Cambridge University Press, Cambridge
Tullis A, Baillie M (2005) The metabolic and biochemical responses of tropical whitespotted bamboo shark Chiloscyilium plagiosum to alterations in environmental temperature. J Fish Biol 67(4):950–968
Van Dijk PCM, Tesch C, Hardewig I, Pörtner HO (1999) Physiological disturbances at critically high temperatures: a comparison between stenothermal Antarctic and eurythermal temperate eelpouts (Zoarcidae). J Exp Biol 202:3611–3621
Wells RMG (1987) Respiration of Antarctic fish from McMurdo sound. Comp Biochem Physiol 88A(3):417–424
Wells RMG, Tetens V, DeVries AL (1984) Recovery from stress following capture and anaesthesia of Antarctic fish: haematology and blood chemistry. J Fish Biol 25:567–576
Wilson RS, Kuchel LJ, Franklin CE, Davison W (2002) Turning up the heat on subzero fish: thermal dependence of sustained swimming in an Antarctic notothenioid. J Thermal Biol 27:381–386
Zakhartsev MV, De Watcher B, Sartori FJ, Pörtner HO, Blust R (2003) Thermal physiology of the common eelpout (Zoarces viviparous). J Comp Physiol 173(B):365–378
Acknowledgments
Authors thank Dr. Cara Lowe and Gavin Robinson for their help with experimental set ups and the staff of Antarctica New Zealand for all of their support in Antarctica and New Zealand. Also thank you to Antarctica New Zealand and Kelly Tarlton’s Underwater World and Antarctic Attraction for their assistance with funding for the research. The experimental protocols were approved by the University of Canterbury Animal Ethics Committee. They would also like to thank the reviewers for their helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robinson, E., Davison, W. The Antarctic notothenioid fish Pagothenia borchgrevinki is thermally flexible: acclimation changes oxygen consumption. Polar Biol 31, 317–326 (2008). https://doi.org/10.1007/s00300-007-0361-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-007-0361-4