Abstract
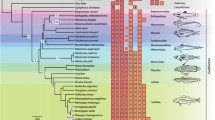
Fishes thriving in polar habitats offer many opportunities for comparative approaches to understanding protein thermal adaptations. Investigations on the remarkable evolutionary adaptations to these environments of basic proteins such as hemoglobin, the oxygen carrier, can provide new insights into the mechanisms studied in temperate organisms and can shed light on convergent processes evolved in response to thermal adaptations. At the molecular level, hemoglobins are one of the most intriguing systems for studying the relationships between environmental conditions and adaptations. This review summarizes the current knowledge on molecular structure, biological function and phylogeny of hemoglobins of fish species living in both polar habitats but having different evolutionary histories. In benthic, non-migratory, cold-adapted fishes, the stability of thermal conditions may have generated no or few variations in selective pressures on globin sequences through evolutionary time, so that sequences retain the species phylogenetic “signal”. In pelagic, migratory, cold-adapted or temperate fishes, variations in selective pressures on globin sequences caused by variations in temperature accompanying the dynamic life style may have disrupted the phylogenetic “signal” in phenetic trees.



Similar content being viewed by others
References
Baldwin J (1980) Structure and cooperativity of hemoglobin. Trends Biochem Sci 5:224–228
Baldwin J, Chothia C (1979) Haemoglobin: structural changes related to ligand binding and its allosteric mechanism. J Mol Biol 129:175–220
Balushkin AV (1992) Classification, phylogenetic relationships, and origins of the families of the suborder Notothenioidei (Perciformes). J Ichthyol 32:90–110
Balushkin AV (2000) Morphology, classification, and evolution of notothenioid fishes of the Southern Ocean (Notothenioidei, Perciformes). J Ichthyol 40:S74–S109
Bargelloni L, Lecointre G (1998) Four years in Notothenioid systematics: a molecular perspective. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Berlin Heidelberg New York, pp 259–273
Bargelloni L, Ritchie PA, Patarnello T, Battaglia B, Lambert DM, Meyer A (1994) Molecular evolution at subzero temperatures: mitochondrial and nuclear phylogenies of fishes from Antarctica (suborder Notothenioidei), and the evolution of antifreeze glycopeptides. Mol Biol Evol 11:854–863
Bargelloni L, Marcato S, Zane LG, Patarnello T (2000a) Mitochondrial phylogeny of Notothenioids: a molecular approach to Antarctic fish evolution and biogeography. Syst Biol 49:114–129
Bargelloni L, Zane L, Derome N, Lecointre G, Patarnello T (2000b) Molecular zoogeography of Antarctic euphausiids and notothenioids: from species phylogenies to intraspecific patterns of genetic variation. Antarctic Sci 12:259–268
Berenbrink M, Koldkjær P, Kepp O, Cossins AR (2005) Evolution of oxygen secretion in fishes and the emergence of a complex physiological system. Science 307:1752–1757
Brittain T (1987) The Root effect. Comp Biochem Physiol 86B:473–481
Chen L, DeVries AL, Cheng C-HC (1997a) Evolution of antifreeze glycoprotein gene from a trypsinogen gene in Antarctic notothenioid fish. Proc Natl Acad Sci USA 94:3811–3816
Chen L, DeVries AL, Cheng C-HC (1997b) Convergent evolution of antifreeze glycoproteins in Antarctic notothenioid fish and Arctic cod. Proc Natl Acad Sci USA 94:3817–3822
Chen WJ, Bonillo C, Lecointre G (1998) Phylogeny of the Channichthyidae (Notothenioidei, teleostei) based on two mitochondrial genes. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Berlin Heidelberg New York, pp 287–298
Cheng C-HC, DeVries AL (1991) The role of antifreeze glycopeptides and peptides in the freezing avoidance of cold-water fish. In: di Prisco G (ed) Life under extreme conditions: biochemical adaptation. Springer, Berlin Heidelberg New York, pp 1–14
Cheng C-HC, Chen L, Near TJ, Jin Y (2003) Functional antifreeze glycoprotein genes in temperate–water New Zealand nototheniid fish infer an Antarctic evolutionary origin. Mol Biol Evol 20:1897–1908
Clarke A, Johnston I (1996) Evolution and adaptive radiation of Antarctic fishes. TREE 11:212–218
D’Avino R, Caruso C, Tamburrini M, Romano M, Rutigliano B, Polverino de Laureto P, Camardella L, Carratore V, di Prisco G (1994) Molecular characterization of the functionally distinct hemoglobins of the Antarctic fish Trematomus newnesi. J Biol Chem 269:9675–9681
Derome N, Chen WJ, Dettaï A, Bonillo C, Lecointre G (2002) Phylogeny of Antarctic dragon fishes (Bathydraconidae, Notothenioidei, Teleostei) and related families based on their anatomy and two mitochondrial genes. Mol Phyl Evol 24:139–152
Dettaï A, Lecointre G (2004) In search for notothenioid (Teleostei) relatives. Antarctic Sci 16:71–85
Dettaï A, Lecointre G (2005) Further support for the clades obtained by multiple molecular phylogenies in the acanthomorph bush. CR Biol 328:674–689
DeWitt HH, Heemstra PC, Gon O (1990) Nototheniidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 279–331
Dickerson RE, Geiss I (1983) Hemoglobin: structure, function, evolution, and pathology. The Benjamin/Cummings Publishing Company, Menlo Park
Eakin RR (1981) Osteology and relationships of the fishes of the Antarctic family Harpagiferidae (Pisces, Notothenioidei). In: Kornicker LS (ed) Biology of the Antarctic Seas. IX. Antarctic Res Series, vol 31. Washington, pp 81–147
Eastman JT (1988) Ocular morphology in Antarctic notothenioid fishes. J Morphol 196:927–934
Eastman JT (1993) Antarctic fish biology: evolution in a unique environment. Academic Press, San Diego
Eastman JT (1997) Comparison of the Antarctic and Arctic fish faunas. Cybium 12:276–287
Eastman JT (2000) Antarctic notothenioid fishes as subjects for research in evolutionary biology. Antarctic Sci 12:276–287
Eastman JT (2005) The nature of the diversity of Antarctic fishes. Polar Biol 28:93–107
Eastman JT, Clarke A (1998) A comparison of adaptive radiations of Antarctic fish with those of non-Antarctic fish. In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Berlin Heidelberg New York, pp 3–26
Eastman JT, McCune AR (2000) Fishes on the Antarctic shelf: evolution of a marine species flock? J Fish Biol 57:84–102
Everson I, Ralph R (1968) Blood analyses of some Antarctic fish. Br Antarctic Surv Bull 15:59–62
Fago A, D’Avino R, di Prisco G (1992) The hemoglobins of Notothenia angustata, a temperate fish belonging to a family largely endemic to the Antarctic Ocean. Eur J Biochem 210:963–970
Gardner PR, Gardner AM, Martin LA, Salzman AL (1998) Nitric oxide dioxygenase: An enzymic function for flavohemoglobin. Proc Natl Acad Sci USA 95:10378–10383
Gelin BR, Lee AW-N, Karplus M (1983) Haemoglobin tertiary structural change on ligand binding. J Mol Biol 171:489–559
Giordano D, Grassi L, Parisi E, Bargelloni L, di Prisco G, Verde C (2006) Embryonic β-globin in the non-Antarctic notothenioid fish Cottoperca gobio (Bovichtidae). Polar Biol (in press)
Grande L, Eastman JT (1986) A review of Antarctic ichthyofaunas in the light of new fossil discoveries. Paleontology 29:113–137
Hardison R (1998) Hemoglobins from bacteria to man: evolution of different patterns of gene expression. J Exp Biol 201:1099–1117
Hastings PA (1993) Relationships of fishes of the perciform suborder Notothenioidei. In: Miller RG (eds) A history and atlas of the fishes of the Antarctic Ocean. Foresta Institute for Ocean and Mountain Studies, Carson City, pp 99–107
Hureau J-C, Petit D, Fine JM, Marneux M (1977) New cytological, biochemical and physiological data on the colorless blood of the Channichthyidae (Pisces, Teleosteans, perciformes). In: Llano GA (eds) Adaptations within Antarctic ecosystems. Smithsonian Institution, Washington, pp 459–477
Imamura H, Yabe M (2002) Demise of the scorpaeniformes (Actinopterygii: Percomorpha): an alternative phylogenetic hypothesis. Bull Fish Sci Hokkaido Univ 53:107–132
Ito N, Komiyama NH, Fermi G (1995) Structure of deoxyhaemoglobin of the Antarctic fish Pagothenia bernacchii with an analysis of the structural basis of the Root effect by comparison of the liganded and unliganded haemoglobin structures. J Mol Biol 250:648–658
Iwami T (1985) Osteology and relationships of the family Channichthyidae. Mem Natl Inst Pol Res Tokyo Ser E 36:1–69
Kennett JP (1982) Marine geology. Prentice Hall, Englewood Cliffs
Lecointre G, Ozouf-Costaz C (2004) Les poissons à antigel de l’océan austral. Pour La Science (in french)
Lecointre G, Bonillo C, Ozouf-Costaz C, Hureau J-C (1997) Molecular phylogeny of the Antarctic fishes: paraphyly of the Bovichtidae and no indication for the monophyly of the Notothenioidei (Teleostei). Polar Biol 18:193–208
Mayr E (1974) Populations, espèces et évolution. Hermann, Paris
Maruyama K, Yasumasu S, Iuchi I (2004) Evolution of globin genes of the medaka Oryzias latipes (Euteleostei; Beloniformes; Oryziinae). Mech Dev 121:753–769
Mazzarella L, D’Avino R, di Prisco G, Savino C, Vitagliano L, Moody PCE, Zagari A (1999) Crystal structure of Trematomus newnesi haemoglobin re-opens the Root effect question. J Mol Biol 287:897–906
Mazzarella L, Bonomi G, Lubrano MC, Merlino A, Riccio A, Vergara A, Vitagliano L, Verde C, di Prisco G (2006) Minimal structural requirements for Root effect: crystal structure of the cathodic hemoglobin isolated from the Antarctic fish Trematomus newnesi. Proteins 62:316–321
Minning DM, Gow AJ, Bonaventura J, Braun R, Dewhirst M, Goldberg DE, Stamler JS (1999) Ascaris haemoglobin is a nitric oxide-activated “deoxygenase”. Nature 401:497–502
Miya M, Takeshima H, Endo H, Ishiguro NB, Inoue JG, Mukai T, Satoh TP, Yamaguchi M, Kawaguchi A, Mabuchi K, Shirai SM, Nishida M (2003) Major patterns of higher teleostean phylogenies: a new perspective based on 100 complete mitochondrial DNA sequences. Mol Phyl Evol 26:121–138
Monod J, Wyman J, Changeux JP (1965) On the nature of allosteric transitions: a plausible model. J Mol Biol 12:88–118
Near T (2004) Estimating divergence times of notothenioid fishes using a fossil-calibrated molecular clock. Antarctic Sci 16:37–44
Near T, Pesavento JJ, Cheng C-HC (2004) Phylogenetic investigations of Antarctic fishes (Perciformes: Notothenioidei) using complete gene sequences of the mitochondrial encoded 16S rRNA. Mol Phyl Evol 32:881–891
Perutz MF (1970) Stereochemistry of cooperative effects in haemoglobin. Nature 228:726–739
Perutz MF (1983) Species adaptation in a protein molecule. Mol Biol Evol 1:1–28
Perutz MF, Fermi G, Luisi B, Shanan B, Liddington RC (1987) Stereochemistry of cooperative mechanisms in hemoglobin. Acc Chem Res 20:309–321
Pisano E, Ozouf-Costaz C, Prirodina V (1998) Chromosome diversification in Antarctic fish (Notothenioidei) In: di Prisco G, Pisano E, Clarke A (eds) Fishes of Antarctica. A biological overview. Springer, Berlin Heidelberg New York, pp 275–285
di Prisco G, Giardina B, D’Avino R, Condò SG, Bellelli A, Brunori M (1988) Antarctic fish hemoglobin: an outline of the molecular structure and oxygen binding properties—II. Oxygen binding properties. Comp Biochem Physiol 90B:585–591
di Prisco G, D’Avino R, Caruso C, Tamburrini M, Camardella L, Rutigliano B, Carratore V, Romano M (1991) The biochemistry of oxygen transport in red-blooded Antarctic fish. In: di Prisco G, Maresca B, Tota B (eds) Biology of Antarctic Fish. Springer, Berlin Heidelberg New York, pp 263–281
Riccio A, Tamburrini M, Carratore V, di Prisco G (2000) Functionally distinct hemoglobins of the cryopelagic Antarctic teleost Pagothenia borchgrevinki. J Fish Biol 57:20–32
Riggs A (1988) The Bohr effect. Annu Rev Physiol 50:181–204
Ritchie PA, Lavoué S, Lecointre G (1997) Molecular phylogenetics and evolution of Antarctic notothenioid fishes. Comp Biochem Physiol 4:1009–1027
Root RW (1931) The respiratory function of the blood of marine fishes. Biol Bull 61:427–456
Root RW, Irving L (1941) The equilibrium between hemoglobin and oxygen in whole and hemolysed blood of the tautog, with a theory of the Haldane effect. Biol Bull 81:307–323
Ruud JT (1954) Vertebrates without erythrocytes and blood pigment. Nature 173:848–850
Sanchez S, Dettaï A, Bonillo C, Ozouf-Costaz C, Detrich HW, Lecointre G (2006) Molecular and morphological phylogenies of the Antarctic teleostean family Nototheniidae, with emphasis on the Trematominae. Polar Biol (in press)
Scholander PF (1954) Secretion of gases against high pressures in the swimbladder of deep sea fishes. II. The rete mirabile. Biol Bull 107:260–277
Stam WT, Beintema JJ, D’Avino R, Tamburrini M, di Prisco G (1997) Molecular evolution of hemoglobins of Antarctic fishes (Notothenioidei). J Mol Evol 45:437–445
Stehmann M, Burkel DL (1990) Rajidae. In: Gon O, Heemstra PC (eds) Fishes of the Southern Ocean. JLB Smith Institute of Ichthyology, Grahamstown, pp 86–97
Tamburrini M, D’Avino R, Fago A, Carratore V, Kunzmann A, di Prisco G (1996) The unique hemoglobin system of Pleuragramma antarcticum, an Antarctic migratory teleost. Structure and function of the three components. J Biol Chem 271:23780–23785
Verde C, di Prisco G (2003) The oxygen transport system of polar fish: the evolution of hemoglobin. Ocean Polar Res 25:617–623
Verde C, di Prisco G (2004) Structure/function and phylogeny of hemoglobins of polar fishes. Micron 35:77–80
Verde C, Carratore V, Riccio A, Tamburrini M, Parisi E, di Prisco G (2002) The functionally distinct hemoglobins of the Arctic spotted wolffish Anarhichas minor. J Biol Chem 277:36312–36320
Verde C, Parisi E, di Prisco G (2003) The evolution of polar fish hemoglobin: a phylogenetic analysis of the ancestral amino acid residues linked to the Root effect. J Mol Evol 57:S258–S267
Verde C, Howes BD, De Rosa MC, Raiola L, Smulevich G, Williams R, Giardina B, Parisi E, di Prisco G (2004a) Structure and function of the Gondwanian hemoglobin of Pseudaphritis urvillii, a primitive notothenioid fish of temperate latitudes. Prot Sci 13:2766–2781
Verde C, Parisi E, di Prisco G (2004b) The evolution of polar fish hemoglobin: structure, function and phylogeny. Antarctic Sci 16:59–69
Verde C, De Rosa MC, Giordano D, Mosca D, de Pascale D, Raiola L, Cocca E, Carratore V, Giardina B, di Prisco G (2005) Structure, function and molecular adaptations of haemoglobins of the polar cartilaginous fish Bathyraja eatonii and Raja hyperborea. Biochem J 389:297–306
Verde C, Balestrieri M, de Pascale D, Pagnozzi D, Lecointre G, di Prisco G (2006) The oxygen-transport system in three species of the boreal fish family Gadidae. Molecular phylogeny of haemoglobin. J Biol Chem 281:22075–22084
Wells RMG, Ashby MD, Duncan SJ, Macdonald JA (1980) Comparative studies of the erythrocytes and haemoglobins in nototheniid fishes from Antarctica. J Fish Biol 17:517–527
Wells RMG, Macdonald JA, di Prisco G (1990) Thin-blooded Antarctic fishes: a rheological comparison of the haemoglobin-free icefishes, Chionodraco kathleenae and Cryodraco antarcticus, with a red-blooded nototheniid, Pagothenia bernacchii. J Fish Biol 36:595–609
Wittenberg JB, Wittenberg BA (1974) The choroid rete mirabile. I. Oxygen secretion and structure: comparison with the swimbladder rete mirabile. Biol Bull 146:116–136
Yonetani T, Park S, Tsuneshige A, Imai K, Kanaori K (2002) Global allostery model of hemoglobin. J Biol Chem 277:34508–34520
Acknowledgments
We thank Professor Dr. G. Hempel for inviting us to write this review. This study is supported by the Italian National Programme for Antarctic Research (PNRA). It is in the framework of the Arctic Strategic Programme of the Italian National Research Council, the SCAR programmes Ecology of the Antarctic Sea Ice Zone (EASIZ), Evolutionary Biology of Antarctic Organisms (EVOLANTA) and Evolution and Biodiversity in the Antarctic (EBA), and the 2003 and 2005 TUNU-I and TUNU-II cruises near Greenland. We also thank the captain, crew and personnel of Raytheon Polar Services aboard the RVIB Nathaniel B. Palmer for their excellent assistance during the ICEFISH cruise. The ICEFISH cruise was supported by National Science Foundation grant OPP 01-32032 to H. William Detrich (Northeastern University, Boston USA).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Verde, C., Lecointre, G. & di Prisco, G. The phylogeny of polar fishes and the structure, function and molecular evolution of hemoglobin. Polar Biol 30, 523–539 (2007). https://doi.org/10.1007/s00300-006-0217-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-006-0217-3