Abstract
The capability of Phalaenopsis to acclimate its photosynthetic capacity and metabolic activity to cool night temperature conditions is crucial for improving orchid production in terms of efficient greenhouse heating. The extent to which Phalaenopsis possesses acclimation potential and the mechanistic background of the metabolic processes involved, have, however, not been studied before. Plants were subjected to a direct and gradual shift from a day to night temperature regime of 28/28–28/16°C, the cold stress and cold acclimation treatment, respectively. In comparison with the cold stress treatment, the cold acclimation treatment led to a higher malate accumulation and a reduction in leaf net CO2 uptake. Consistently, the contribution of respiratory CO2 recycling to nocturnal malate synthesis was calculated to be 23.5 and 47.0% for the cold stress and cold acclimation treatment, respectively. Moreover, the lower levels of starch measured in the cold acclimated leaves confirmed the suggested enhanced respiratory CO2 recycling, implying that Phalaenopsis CAM operation evolved towards CAM idling. It is, however, plausible that this adjustment was not an effect of the low night temperature per se but a consequence of cool-root induced drought stress. Apart from that, at the start of the photoperiod, membrane stability showed a depression which was directly counteracted by an increased generation of glucose, fructose and sucrose. From these observations, it can be concluded that the observed plasticity in CAM operation and metabolic flexibility may be recognized as important steps in the low night temperature acclimation of Phalaenopsis.





Similar content being viewed by others
Abbreviations
- ARS:
-
Antioxidative response system
- CA:
-
Cold acclimation
- CAM:
-
Crassulacean acid metabolism
- CS:
-
Cold stress
- EC:
-
Electrical conductivity
- F0′:
-
Minimal fluorescence from the light-adapted state
- F m :
-
Maximal fluorescence at the dark-adapted state
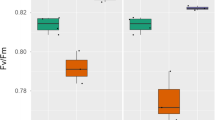
- Fv/Fm:
-
Maximum quantum efficiency of PSII photochemistry
- Fq′/Fm′:
-
PSII operating efficiency
- Fq′/Fv′:
-
PSII efficiency factor
- NPQ:
-
Non-photochemical quenching
- PAR:
-
Photosynthetic active radiation
- PEPC:
-
Phosphoenolpyruvate carboxylase
References
Allen DJ, Ort DR (2001) Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci 6:36–42
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Bartok JJ (2001) Energy conservation for commercial greenhouses. Natural Resource, Agriculture, and Engineering Service Cooperative Extension, Ithaca
Blanchard M, Runkle ES (2006) Temperature during the day, but not during the night, controls flowering of Phalaenopsis orchids. J Exp Bot 57:4043–4049
Boese SR, Huner NPA (1990) Effect of growth temperature and temperature shifts on spinach leaf morphology and photosynthesis. Plant Physiol 94:1830–1836
Borland AM, Elliott S, Patterson S, Taybi T, Cushman J, Pater B, Barnes J (2006) Are the metabolic components of Crassulacean acid metabolism up-regulated in response to an increase in oxidative burden? J Exp Bot 57:319–328
Buchanan-Bollig IC, Kluge M (1981) Crassulacean acid metabolism (CAM) in Kalanchoe daigremontiana—temperature response of phosphoenolpyruvate (PEP)-carboxylase in relation to allosteric effectors. Planta 152:181–188
Buchanan-Bollig IC, Kluge M, Muller D (1984) Kinetic changes with temperature of phosphoenolpyruvate carboxylase from a CAM Plant. Plant Cell Environ 7:63–70
Buwalda F, Eveleens B, Wertwijn R (2000) Ornamental crops tolerate large temperature fluctuations: a potential for more efficient greenhouse heating strategies. Acta Hort 515:141–149
Carter PJ, Wilkins MB, Nimmo HG, Fewson CA (1995) Effects of temperature on the activity of phosphoenolpyruvate carboxylase and on the control of CO2 fixation in Bryophyllum fedtschenkoi. Planta 196:375–380
Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP (2009) Differential usage of storage carbohydrates in the CAM bromeliad Aechmea ‘Maya’ during acclimation to drought and recovery from dehydration. Physiol Plant 135:174–184
Chen WS, Liu HY, Liu ZH, Yang L, Chen WH (1994) Gibberellin and temperature influence carbohydrate content and flowering in Phalaenopsis. Physiol Plant 90:391–395
Chen WH, Tseng YC, Liu YC, Chuo CM, Chen PT, Tseng KM, Yeh YC, Ger MJ, Wang HL (2008) Cool-night temperature induces spike emergence and affects photosynthetic efficiency and metabolizable carbohydrate and organic acid pools in Phalaenopsis aphrodite. Plant Cell Rep 27:1667–1675
Crookston RK, Otoole J, Lee R, Ozbun JL, Wallace DH (1974) Photosynthetic depression in beans after exposure to cold for one night. Crop Sci 14:457–464
Dodd AN, Borland AM, Haslam RP, Griffiths H, Maxwell K (2002) Crassulacean acid metabolism: plastic, fantastic. J Exp Bot 53:569–580
Endo M, Ikusima I (1989) Diurnal rhythm and characteristics of photosynthesis and respiration in the leaf and root of a Phalaenopsis plant. Plant Cell Physiol 30:43–47
Griesbach RJ (2000) Potted Phalaenopsis orchid production: history, present status, and challenges for the future. HortTechnology 10:429
Guo WJ, Lee N (2006) Effect of leaf and plant age, and day/night temperature on net CO2 uptake in Phalaenopsis amabilis var. formosa. J Am Soc Hortic Sci 131:320–326
Haag-Kerwer A, Franco AC, Luttge U (1992) The effect of temperature and light on gas exchange and acid accumulation in the C3/CAM plant Clusia minor L. J Exp Bot 43:345–352
Herppich WB, Herppich M, von Willert DJ (1998) Ecophysiological investigations on plants of the genus Plectranthus (Lamiaceae). Influence of environment and leaf age on CAM, gas exchange and leaf water relations in Plectranthus marrubioides Benth. Flora 193:99–109
Hew CS, Yong JWH (1997) The physiology of tropical orchids in relation to the industry. World Scientific, Singapore
Ichihashi S, Higuchi T, Shibayama H, Tesima Y, Nishiwaki Y, Ota K (2008) Aspects of CO2 uptake in the Crassulacean acid metabolism orchid Phalaenopsis. Acta Hort 766:245–256
Janská A, Marsik P, Zelenková S, Ovesná J (2010) Cold stress and acclimation—what is important for metabolic adjustment? Plant Biol 12:395–405
Kacperska A (1999) Plant response to low temperature: signaling pathways involved in plant acclimation. In: Margesin R, Schinner F (eds) Cold-adapted organisms—ecology, physiology, enzymology and molecular biology. Springer, Berlin, pp 79–103
Kingston-Smith AH, Harbinson J, Foyer CH (1999) Acclimation of photosynthesis, H2O2 content and antioxidants in maize (Zea mays) grown at sub-optimal temperatures. Plant Cell Environ 22:1071–1083
Körner O, Bakker MJ, Heuvelink E (2004) Daily temperature integration: a simulation study to quantify energy consumption. Biosyst Eng 87:333–343
Krol M, Griffith M, Huner NPA (1984) An appropriate physiological control for environmental temperature studies: comparative growth kinetics of winter rye. Can J Bot Rev Can Bot 62:1062–1068
Livingston DP, Premakumar R, Tallury SP (2006) Carbohydrate partitioning between upper and lower regions of the crown in oat and rye during cold acclimation and freezing. Cryobiology 52:200–208
Lootens P, Heursel J (1998) Irradiance, temperature, and carbon dioxide enrichment affect photosynthesis in Phalaenopsis hybrids. Hortscience 33:1183–1185
Lund JB, Andreassen A, Ottosen CO, Aaslyng JM (2006) Effect of a dynamic climate on energy consumption and production of Hibiscus rosa-sinensis L. in greenhouses. Hortscience 41:384–388
Lüttge U (1988) Day-night changes of citric acid levels in Crassulacean acid metabolism: phenomenon and ecophysiological significance. Plant Cell Environ 11:445–451
Lüttge U (1990) Nocturnal citrate accumulation and its response to environmental stress in the CAM plant Kalanchoe pinnata Lam. Pers. Plant Cell Environ 13:977–982
Lüttge U (2002) CO2-concentrating: consequences in Crassulacean acid metabolism. J Exp Bot 53:2131–2142
Lüttge U (2004) Ecophysiology of Crassulacean acid metabolism (CAM). Ann Bot 93:629–652
Lüttge U (2006) Photosynthetic flexibility and ecophysiological plasticity: questions and lessons from Clusia, the only CAM tree, in the neotropics. New Phytol 171:7–25
Nimmo HG (2000) The regulation of phosphoenolpyruvate carboxylase in CAM plants. Trends Plant Sci 5:75–80
Osmond CB (1978) Crassulacean acid metabolism—curiosity in context. Annu Rev Plant Phys 29:379–414
Ota K, Morioka K, Yamamoto Y (1991) Effects of leaf age, inflorescence, temperature, light-intensity and moisture conditions on CAM photosynthesis in Phalaenopsis. J Jpn Soc Hort Sci 60:125–132
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and Fv′/Fm′ without measuring F0′. Photosynth Res 54:135–142
Pollet B, Steppe K, Van Labeke MC, Lemeur R (2009) Diurnal cycle of chlorophyll fluorescence in Phalaenopsis. Photosynthetica 47:309–312
Pollet B, Steppe K, Dambre P, Van Labeke MC, Lemeur R (2010) Seasonal variation of photosynthesis and photosynthetic efficiency in Phalaenopsis. Photosynthetica 48:580–588
Pollet B, Kromwijk A, Vanhaecke L, Dambre P, Van Labeke MC, Marcelis LFM, Steppe K (2011) A new method to determine the optimal night temperature for vegetative growth of Phalaenopsis. Ann Appl Biol (accepted)
Pridgeon A (2000) The illustrated encyclopedia of orchids. Timber Press, Portland
Sayed OH (2001) Crassulacean acid metabolism 1975–2000, a check list. Photosynthetica 39:339–352
Singh M, Johnson-Flanagan A (1998) Co-ordination of photosynthetic gene expression during low-temperature acclimation and development in Brassica napus cv. Jet Neuf leaves. Plant Sci 135:171–181
Smirnoff N (1993) The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol 125:27–58
Szarek SR, Johnson HB, Ting IP (1973) Drought adaptation in Opuntia basilaris. Significance of recycling carbon through Crassulacean acid metabolism. Plant Physiol 52:539–541
Wang YT (2007) Average daily temperature and reversed day/night temperature regulate vegetative and reproductive responses of a Doritis pulcherrima Lindley hybrid. Hortscience 42:68–70
Winter K, Schröppel-Meier G, Caldwell MM (1986) Respiratory CO2 as carbon source for nocturnal acid synthesis at high temperatures in 3 species exhibiting Crassulacean acid metabolism. Plant Physiol 81:390–394
Wu MT, Wallner SJ (1983) Heat stress responses in cultured plant cells: development and comparison of viability tests. Plant Physiol 72:817–820
Acknowledgments
This research was partly funded by the Ministry of Flemish Community (IWT-project LANDBOUW 60667/PCS). The authors would like to thank Philip Deman and Geert Favyts of the Laboratory of Plant Ecology and Benny De Geest of Research Centre for Ornamental Plants for technical assistance. The authors are also very grateful to Dirk Stockx and Vera Paltousova for their assistance in the lab at the Laboratory of Chemical Analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Altman.
Rights and permissions
About this article
Cite this article
Pollet, B., Vanhaecke, L., Dambre, P. et al. Low night temperature acclimation of Phalaenopsis . Plant Cell Rep 30, 1125–1134 (2011). https://doi.org/10.1007/s00299-011-1021-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-011-1021-2