Abstract
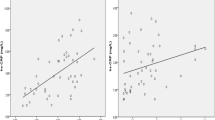
The aim of the study was to measure the diagnostic values of biomarkers of bacterial infection in idiopathic inflammatory myopathy (IIM) patients. The serum and clinical data of 82 IIM patients with/without bacterial infection were collected. Concentrations of soluble urokinase plasminogen activator receptor (suPAR), soluble triggering receptor expressed on myeloid cells 1 (sTREM-1), procalcitonin (PCT) and C-reactive protein (CRP) were measured in IIM patients and healthy controls. There were no significant differences in serum suPAR and sTREM-1 levels between healthy controls and non-infection IIM patients. Serum levels of suPAR, sTREM-1, PCT and CRP measured in this study were significantly higher in the IIM patient group with concurrent infection than in the non-infection IIM patient group (p < 0.05). The biomarker suPAR showed the highest diagnostic value with sensitivity, specificity, positive predictive value and negative predictive value of 81.6, 77.3, 75.6 and 82.9%, respectively. Combining suPAR negative and CRP negative to rule out bacterial infection in IIM patients provides a very high specificity of 97.4%. Both suPAR and CRP positive to confirm bacterial infection give the specificity of 90.9%. The inflammatory biomarkers suPAR, sTREM-1, PCT and CRP offer diagnostic accuracy in detecting bacterial infection in IIM patients. Particularly, suPAR is the most sensitive and specific biomarker to predict bacterial infection in IIM patients. Combination of suPAR and CRP serum levels provides an even better confirmation of bacterial infection.



Similar content being viewed by others
References
Ernste FC, Reed AM (2013) Idiopathic inflammatory myopathies: current trends in pathogenesis, clinical features, and up-to-date treatment recommendations. Mayo Clin Proc 88:83–105. doi:10.1016/j.mayocp.2012.10.017
Torres C, Belmonte R, Carmona L, Gomez-Reino FJ, Galindo M, Ramos B et al (2006) Survival, mortality and causes of death in inflammatory myopathies. Autoimmunity 39:205–215. doi:10.1080/08916930600622603
Maldonado F, Patel RR, Iyer VN, Yi ES, Ryu JH (2012) Are respiratory complications common causes of death in inflammatory myopathies? An autopsy study. Respirology 17:455–460. doi:10.1111/j.1440-1843.2011.02103.x
Sciascia S, Ceberio L, Garcia-Fernandez C, Roccatello D, Karim Y, Cuadrado MJ (2012) Systemic lupus erythematosus and infections: clinical importance of conventional and upcoming biomarkers. Autoimmun Rev 12:157–163. doi:10.1016/j.autrev.2012.03.009
Cho SY, Choi JH (2014) Biomarkers of sepsis. Infect Chemother 46:1–12. doi:10.3947/ic.2014.46.1.1
Buhaescu I, Yood RA, Izzedine H (2010) Serum procalcitonin in systemic autoimmune diseases–where are we now? Semin Arthritis Rheum 40:176–183. doi:10.1016/j.semarthrit.2009.10.004
Bador KM, Intan S, Hussin S, Gafor AH (2012) Serum procalcitonin has negative predictive value for bacterial infection in active systemic lupus erythematosus. Lupus 21:1172–1177. doi:10.1177/0961203312450085
Wu JY, Lee SH, Shen CJ, Hsieh YC, Yo PH, Cheng HY et al (2012) Use of serum procalcitonin to detect bacterial infection in patients with autoimmune diseases: a systematic review and meta-analysis. Arthritis Rheum 64:3034–3042. doi:10.1002/art.34512
Ploug M, Ronne E, Behrendt N, Jensen AL, Blasi F, Dano K (1991) Cellular receptor for urokinase plasminogen activator. Carboxyl-terminal processing and membrane anchoring by glycosyl-phosphatidylinositol. J Biol Chem 266:1926–1933
Ford JW, McVicar DW (2009) TREM and TREM-like receptors in inflammation and disease. Curr Opin Immunol 21:38–46. doi:10.1016/j.coi.2009.01.009
Gibot S (2005) Clinical review: role of triggering receptor expressed on myeloid cells-1 during sepsis. Crit Care 9:485–489. doi:10.1186/cc3732
Bouchon A, Facchetti F, Weigand MA, Colonna M (2001) TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410:1103–1107. doi:10.1038/35074114
Prucha M, Bellingan G, Zazula R (2015) Sepsis biomarkers. Clin Chim Acta 440:97–103. doi:10.1016/j.cca.2014.11.012
Backes Y, van der Sluijs KF, Mackie DP, Tacke F, Koch A, Tenhunen JJ et al (2012) Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med 38:1418–1428. doi:10.1007/s00134-012-2613-1
Zhang J, She D, Feng D, Jia Y, Xie L (2011) Dynamic changes of serum soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) reflect sepsis severity and can predict prognosis: a prospective study. BMC Infect Dis 11:53. doi:10.1186/1471-2334-11-53
Jiyong J, Tiancha H, Wei C, Huahao S (2009) Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: a meta-analysis. Intensive Care Med 35:587–595. doi:10.1007/s00134-008-1333-z
Gibot S, Kolopp-Sarda MN, Bene MC, Cravoisy A, Levy B, Faure GC et al (2004) Plasma level of a triggering receptor expressed on myeloid cells-1: its diagnostic accuracy in patients with suspected sepsis. Ann Intern Med 141:9–15
Bohan A, Peter JB (1975) Polymyositis and dermatomyositis (first of two parts). N Engl J Med 292:344–347. doi:10.1056/NEJM197502132920706
Furst DE, Amato AA, Iorga SR, Gajria K, Fernandes AW (2012) Epidemiology of adult idiopathic inflammatory myopathies in a U.S. managed care plan. Muscle Nerve 45:676–683. doi:10.1002/mus.23302
Murray SG, Schmajuk G, Trupin L, Lawson E, Cascino M, Barton J et al (2015) A population-based study of infection-related hospital mortality in patients with dermatomyositis/polymyositis. Arthritis Care Res (Hoboken) 67:673–680. doi:10.1002/acr.22501
Yu J, Xu B, Huang Y, Zhao J, Wang S, Wang H et al (2014) Serum procalcitonin and C-reactive protein for differentiating bacterial infection from disease activity in patients with systemic lupus erythematosus. Mod Rheumatol 24:457–463. doi:10.3109/14397595.2013.844391
Scire CA, Cavagna L, Perotti C, Bruschi E, Caporali R, Montecucco C (2006) Diagnostic value of procalcitonin measurement in febrile patients with systemic autoimmune diseases. Clin Exp Rheumatol 24:123–128
Sato H, Tanabe N, Murasawa A, Otaki Y, Sakai T, Sugaya T et al (2012) Procalcitonin is a specific marker for detecting bacterial infection in patients with rheumatoid arthritis. J Rheumatol 39:1517–1523. doi:10.3899/jrheum.111601
Chen DY, Chen YM, Ho WL, Chen HH, Shen GH, Lan JL (2009) Diagnostic value of procalcitonin for differentiation between bacterial infection and non-infectious inflammation in febrile patients with active adult-onset Still’s disease. Ann Rheum Dis 68:1074–1075. doi:10.1136/ard.2008.098335
Moosig F, Csernok E, Reinhold-Keller E, Schmitt W, Gross WL (1998) Elevated procalcitonin levels in active Wegener’s granulomatosis. J Rheumatol 25:1531–1533
Kim HA, Jeon JY, An JM, Koh BR, Suh CH (2012) C-reactive protein is a more sensitive and specific marker for diagnosing bacterial infections in systemic lupus erythematosus compared to S100A8/A9 and procalcitonin. J Rheumatol 39:728–734. doi:10.3899/jrheum.111044
Liu W, Sigdel KR, Wang Y, Su Q, Huang Y, Zhang YL et al (2015) High Level Serum Procalcitonin Associated Gouty Arthritis Susceptibility: from a Southern Chinese Han Population. PLoS ONE 10:e0132855. doi:10.1371/journal.pone.0132855
Marioli A, Koupetori M, Raftogiannis M, Patrani M, Antonakos N, Pavlaki M et al (2014) Early changes of the kinetics of monocyte trem-1 reflect final outcome in human sepsis. BMC Immunol 15:585. doi:10.1186/s12865-014-0063-y
Grover V, Pantelidis P, Soni N, Takata M, Shah PL, Wells AU et al (2014) A biomarker panel (Bioscore) incorporating monocytic surface and soluble TREM-1 has high discriminative value for ventilator-associated pneumonia: a prospective observational study. PLoS ONE 9:e109686. doi:10.1371/journal.pone.0109686
Su L, Han B, Liu C, Liang L, Jiang Z, Deng J et al (2012) Value of soluble TREM-1, procalcitonin, and C-reactive protein serum levels as biomarkers for detecting bacteremia among sepsis patients with new fever in intensive care units: a prospective cohort study. BMC Infect Dis 12:157. doi:10.1186/1471-2334-12-157
Savva A, Raftogiannis M, Baziaka F, Routsi C, Antonopoulou A, Koutoukas P et al (2011) Soluble urokinase plasminogen activator receptor (suPAR) for assessment of disease severity in ventilator-associated pneumonia and sepsis. J Infect 63:344–350. doi:10.1016/j.jinf.2011.07.016
Huttunen R, Syrjanen J, Vuento R, Hurme M, Huhtala H, Laine J et al (2011) Plasma level of soluble urokinase-type plasminogen activator receptor as a predictor of disease severity and case fatality in patients with bacteraemia: a prospective cohort study. J Intern Med 270:32–40. doi:10.1111/j.1365-2796.2011.02363.x
Kim J, Koh JK, Lee EY, Park JA, Kim HA, Lee EB et al (2009) Serum levels of soluble triggering receptor expressed on myeloid cells-1 (sTREM-1) and pentraxin 3 (PTX3) as markers of infection in febrile patients with systemic lupus erythematosus. Clin Exp Rheumatol 27:773–778
Qin DD, Song D, Huang J, Yu F, Zhao MH (2015) Plasma-soluble urokinase-type plasminogen activator receptor levels are associated with clinical and pathological activities in lupus nephritis: a large cohort study from China. Lupus 24:546–557. doi:10.1177/0961203314558857
Legany N, Toldi G, Distler JH, Beyer C, Szalay B, Kovacs L et al (2015) Increased plasma soluble urokinase plasminogen activator receptor levels in systemic sclerosis: possible association with microvascular abnormalities and extent of fibrosis. Clin Chem Lab Med 53:1799–1805. doi:10.1515/cclm-2015-0079
Manetti M, Rosa I, Milia AF, Guiducci S, Carmeliet P, Ibba-Manneschi L et al (2014) Inactivation of urokinase-type plasminogen activator receptor (uPAR) gene induces dermal and pulmonary fibrosis and peripheral microvasculopathy in mice: a new model of experimental scleroderma? Ann Rheum Dis 73:1700–1709. doi:10.1136/annrheumdis-2013-203706
Enocsson H, Wettero J, Skogh T, Sjowall C (2013) Soluble urokinase plasminogen activator receptor levels reflect organ damage in systemic lupus erythematosus. Transl Res 162:287–296. doi:10.1016/j.trsl.2013.07.003
Pliyev BK, Menshikov MY (2010) Release of the soluble urokinase-type plasminogen activator receptor (suPAR) by activated neutrophils in rheumatoid arthritis. Inflammation 33:1–9. doi:10.1007/s10753-009-9152-0
Kaya S, Koksal I, Mentese A, Sonmez M, Sumer A, Yildirim SS et al (2013) The significance of serum urokinase plasminogen activation receptor (suPAR) in the diagnosis and follow-up of febrile neutropenic patients with hematologic malignancies. Int J Infect Dis 17:e1056–e1059. doi:10.1016/j.ijid.2013.04.004
Koch A, Voigt S, Kruschinski C, Sanson E, Duckers H, Horn A et al (2011) Circulating soluble urokinase plasminogen activator receptor is stably elevated during the first week of treatment in the intensive care unit and predicts mortality in critically ill patients. Crit Care 15:R63. doi:10.1186/cc10037
Funding
This work was supported by Hunan Provincial Natural Science Foundation of China (Grant No. 12JJ3106), Hunan Development and Reform Commission of China (Grant No.[2012] 1493) and Central South University of China (Grant No. 2012QNZT106).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yizhi Xiao, Hui Luo, Bin Zhou, Xiaodan Dai, Jing Huang, Liping Duan, Yunhui You, Weiru Zhang, Hongjun Zhao, Yanli Xie, Yaou Zhou, Wangbin Ning, Tong Li, Sijia Liu, Honglin Zhu, Xiaoyun Xie, Ying Jiang, Xiaoyun Xie, Shiyao Wu, Weijia He and Yisha Li declare that they have no conflict of interest.
Human rights
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki Declaration and its later amendments. Ethical approval was received from the Medical Ethics Committee of Xiangya Hospital, Central South University.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Xiao, Y., Luo, H., Zhou, B. et al. Comparison of soluble urokinase plasminogen activator receptor, soluble triggering receptor expressed on myeloid cells 1, procalcitonin and C-reactive protein in distinguishing concurrent bacterial infection from idiopathic inflammatory myopathy. Rheumatol Int 37, 585–592 (2017). https://doi.org/10.1007/s00296-016-3609-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-016-3609-x