Abstract
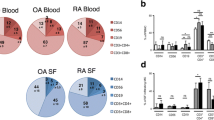
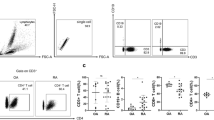
Behçet’s disease (BD) is a multi-system inflammatory disorder, in which cytokine balance is polarized to Th1. In this study, the cell surface molecule expression, Th1/Th2, inflammatory cytokine levels in blood, and synovial fluid of CD3+ T lymphocytes in BD were investigated. The study group consisted of 10 BD, 10 ankylosing spondylitis (AS) patients with peripheral arthritis, and 10 healthy subjects. Expression of cell surface molecules, intracellular IL-2, IL-5, IL-8, IL-10, IL-12, IFN-γ, and TNF-α levels in CD3+ T lymphocytes were determined by flow cytometry in synovial and peripheral blood mononuclear cells (PBMCs). Synovial and plasma cytokine levels were measured by ELISA and CBA. In PBMCs, CD4, CD25, HLA-DR expression and intracellular IL-12, and TNF-α levels of CD3+ T lymphocytes were statistically increased in BD patients compared to healthy subjects. Compare to AS patients, CD25 and HLA-DR surface expression and intracellular IFN-γ and TNF-α levels in T cells were significantly elevated in BD patients. In BD patients, there was an increase in IL-8 secretion; however, in AS patients, both Th1- and Th2-type cytokines were increased compare to healthy subjects. Intracellular cytokine expression did not show any difference in BD patients; however, IL-12 content of synovial fluid was significantly increased compared to AS patients. Our findings revealed that Th1 polarization occurred in both peripheral blood and synovial fluid of BD patients with arthritis. It is found no difference between synovial fluid analysis of BD and AS patients, showing the similarities in the pathogenesis of both diseases.






Similar content being viewed by others
References
The International Study Group for Behçet’s Disease (1992) Evaluation of diagnostic (‘classification’) criteria in Behçet’s disease—towards internationally agreed criteria. Br J Rheumatol 31:299–308
Kaneko F, Togashi A, Saito S et al (2011) Behçet’s disease (Adamantiades-Behçet’s disease). Clin Dev Immunol 2011:681956
Esin S, Gül A, Hodara V, Jeddi-Tehrani M, Dilsen N, Koniçe M, Andersson R, Wigzell H (1997) Peripheral blood T cell expansions in patients with Behcet’s disease. Clin Exp Immunol 107:520–527
Kim J, Park JA, Lee EY, Lee YJ, Song YW, Lee EB (2010) Imbalance of Th17 to Th1 cells in Behçet’s disease. Clin Exp Rheumatol 28:S16–S19
Ilhan F, Demir T, Türkçüoğlu P, Turgut B, Demir N, Gödekmerdan A (2008) Th1 polarization of the immune response in uveitis in Behçet’s disease. Can J Ophthalmol 43:105–108
Rose NR, Bona C (1993) Defining criteria for autoimmune diseases (Witebsky’s postulates revisited). Immunol Today 14:426–430
Harrington LE, Hatton RD, Mangan PR et al (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 6:1123–1132
Moll C, Bogas M, Gómez-Puerta JA et al (2009) Macroscopic features of knee synovitis in early untreated Behçet disease and psoriatic arthritis. Clin Rheumatol 28:1053–1057
Cañete JD, Celis R, Noordenbos T et al (2009) Distinct synovial immunopathology in Behçet disease and psoriatic arthritis. Arthritis Res Ther 11:R17
Amberger M, Groll S, Günaydin I, Deuter C, Vonthein R, Kötter I (2007) Intracellular cytokine patterns in Behçet’s disease in comparison to ankylosing spondylitis–influence of treatment with interferon-alpha2a. Clin Exp Rheumatol 25:S52–S57
Dalghous AM, Freysdottir J, Fortune F (2006) Expression of cytokines, chemokines, and chemokine receptors in oral ulcers of patients with Behcet’s disease (BD) and recurrent aphthous stomatitis is Th1-associated, although Th2-association is also observed in patients with BD. Scand J Rheumatol 35:472–475
Charteris DG, Barton K, McCartney AC, Lightman SL (1992) CD4+ lymphocyte involvement in ocular Behçet’s disease. Autoimmunity 12:201–206
Mochizuki M, Morita E, Yamamoto S, Yamana S (1997) Characteristics of T cell lines established from skin lesions of Behçet’s disease. J Dermatol Sci 15:9–13
Gül A, Esin S, Dilsen N, Koniçe M, Wigzell H, Biberfeld P (1995) Immunohistology of skin pathergy reaction in Behçet’s disease. Br J Dermatol 132:901–907
Sugi-Ikai N, Nakazawa M, Nakamura S, Ohno S, Minami M (1998) Increased frequencies of interleukin-2- and interferon-gamma-producing T cells in patients with active Behçet’s disease. Invest Ophthalmol Vis Sci 39:996–1004
Schroder K, Hertzog PJ, Ravasi T, Hume DA (2004) Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol 75:163–189
Akdis M, Burgler S, Crameri R et al (2011) Interleukins, from 1 to 37, and interferon-γ: receptors, functions, and roles in diseases. J Allergy Clin Immunol 127(3):701-21.e1-70
Raziuddin S, al-Dalaan A, Bahabri S, Siraj AK, al-Sedairy S (1998) Divergent cytokine production profile in Behçet’s disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatol 25:329–333
Sayinalp N, Ozcebe OI, Ozdemir O, Haznedaroğlu IC, Dündar S, Kirazli S (1996) Cytokines in Behçet’s disease. J Rheumatol 23:321–322
Evereklioglu C, Er H, Türköz Y, Cekmen M (2002) Serum levels of TNF-alpha, sIL-2R, IL-6, and IL-8 are increased and associated with elevated lipid peroxidation in patients with Behçet’s disease. Mediators Inflamm 11:87–93
Alpsoy E, Cayirli C, Er H, Yilmaz E (1998) The levels of plasma interleukin-2 and soluble interleukin-2R in Behçet’s disease: a marker of disease activity. J Dermatol 25:513–516
Ertenli I, Kiraz S, Calgüneri M et al (2001) Synovial fluid cytokine levels in Behçet’s disease. Clin Exp Rheumatol 19:S37–S41
Aridogan BC, Yildirim M, Baysal V, Inaloz HS, Baz K, Kaya S (2003) Serum Levels of IL-4, IL-10, IL-12, IL-13 and IFN-gamma in Behçet’s disease. J Dermatol 30:602–607
Nagalakshmi ML, Murphy E, McClanahan T, de Waal Malefyt R (2004) Expression patterns of IL-10 ligand and receptor gene families provide leads for biological characterization. Int Immunopharmacol 4:577–592
de Waal Malefyt R, Haanen J, Spits H et al (1991) Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med 174:915–924
Mizuki N, Meguro A, Ota M et al (2010) Genome-wide association studies identify IL23R-IL12RB2 and IL10 as Behçet’s disease susceptibility loci. Nat Genet 42:703–706
Remmers EF, Cosan F, Kirino Y et al (2010) Genome-wide association study identifies variants in the MHC class I, IL10, and IL23R-IL12RB2 regions associated with Behçet’s disease. Nat Genet 42:698–702
Mukaida N (2000) Interleukin-8: an expanding universe beyond neutrophil chemotaxis and activation. Int J Hematol 72:391–398
Durmazlar SP, Ulkar GB, Eskioglu F, Tatlican S, Mert A, Akgul A (2009) Significance of serum interleukin-8 levels in patients with Behcet’s disease: high levels may indicate vascular involvement. Int J Dermatol 48:259–264
Freire Ade L, Bertolo MB, de Pinho AJ, Samara AM Jr, Fernandes SR (2004) Increased serum levels of interleukin-8 in polyarteritis nodosa and Behçet’s disease. Clin Rheumatol 23:203–205
Zouboulis CC, Katsantonis J, Ketteler R et al (2000) Adamantiades-Behçet’s disease: interleukin-8 is increased in serum of patients with active oral and neurological manifestations and is secreted by small vessel endothelial cells. Arch Dermatol Res 292:279–284
Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP (1999) Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol 17:331–367
Ahn JK, Yu HG, Chung H, Park YG (2006) Intraocular cytokine environment in active Behçet uveitis. Am J Ophthalmol 142:429–434
Oztas MO, Onder M, Gurer MA, Bukan N, Sancak B (2005) Serum interleukin 18 and tumour necrosis factor-alpha levels are increased in Behcet’s disease. Clin Exp Dermatol 30:61–63
Pay S, Erdem H, Pekel A et al (2006) Synovial proinflammatory cytokines and their correlation with matrix metalloproteinase-3 expression in Behçet’s disease. Does interleukin-1beta play a major role in Behçet’s synovitis? Rheumatol Int 26:608–613
Turan B, Pfister K, Diener PA et al (2008) Soluble tumour necrosis factor receptors sTNFR1 and sTNFR2 are produced at sites of inflammation and are markers of arthritis activity in Behçet’s disease. Scand J Rheumatol 37:135–141
Acknowledgments
This work was supported by the Research Fund of The University of Istanbul.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aktas Cetin, E., Cosan, F., Kucuksezer, U.C. et al. Behçet’s disease: immunological relevance with arthritis of ankylosing spondylitis. Rheumatol Int 33, 733–741 (2013). https://doi.org/10.1007/s00296-012-2446-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-012-2446-9