Abstract
Objective
This study aimed to assess the role of synovial fluid (SF) CD4+T, CD19+B, follicular helper cells (Tfh), and cytokines in the pathogenesis of rheumatoid arthritis (RA).
Methods
This study enrolled 16 patients with RA and 8 patients with osteoarthritis (OA). The frequencies of the SF CD4+ T, CD19+ B, Tfh cells, and Tfh subsets were assessed using flow cytometry. The medical condition in patients with RA was evaluated using The Disease Activity Score 28 (DAS28), the Clinical Disease Activity Index (CDAI), and the Simplified Disease Activity Index (SDAI). Levels of C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), anti-cyclic citrullinated peptide (anti-CCP), and rheumatoid factor (RF) were measured. The cytokines IL-4, IL-13, IL-21, and BLyS were measured by ELISA test.
Results
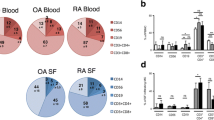
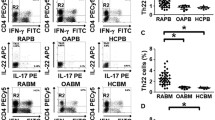
The percentages of SF CD4+T, CD19+B, and PD-1+CXCR5+ Tfh in RA patients were higher than those in OA patients. And the Tfh2 was the main subset among Tfh subsets. In addition, levels of IL-21 and BLyS were higher in patients with RA compared to patients with OA. Furthermore, the treatment of TNF-α inhibitors may be associated with decreased levels of SF Tfh.
Conclusions
Elevated SF Tfh, B cell, and cytokines expression profiles were observed in RA patients. Tfh2 was the major subset of the Tfh, and IL-21 and BLyS were significantly enhanced. Additionally, TNF-α inhibitors reduced Tfh in SF. Therefore, Tfh, B, and Tfh2 cells could play a significant role in the progression of RA.
Key Points •Tfh cells in the synovial fluid are significantly higher in RA patients and are dominated by the Tfh2 subpopulation. •Synovial fluid Tfh cells decrease in RA patients after anti-TNF-α treatment. |





Similar content being viewed by others
References
Gibofsky A et al (2012) Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis[J]. Am J Manag Care. 18(13 Suppl):S295-302
Mcinnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis[J]. N Engl J Med 365(23):2205–2219
Orr C, Sousa E, Boyle DL et al (2017) Synovial tissue research: a state-of-the-art review[J]. Nat Rev Rheumatol 13(8):463–475
Wolfe F, Mitchell DM, Sibley JT et al (2014) The mortality of rheumatoid arthritis[J]. Arthritis Rheum 37(4):481–494
Fekete A, Soos L, Szekanecz Z et al (2007) Disturbances in B- and T-cell homeostasis in rheumatoid arthritis: suggested relationships with antigen-driven immune responses[J]. J Autoimmun 29(2–3):154–163
Choy EH, Panayi GS (2001) Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 344(12):907–916
Deng J, Wei Y, Fonseca VR et al (2019) T follicular helper cells and T follicular regulatory cells in rheumatic diseases[J]. Nat Rev Rheumatol 15(8):475–490
Cannons JL, Lu KT, Schwartzberg PL (2013) T follicular helper cell diversity and plasticity[J]. Trends Immunol 34(5):200–207
Kerschbaumer A, Sepriano A, Smolen JS, van der Heijde D, Dougados M, van Vollenhoven R, McInnes IB, Bijlsma JWJ, Burmester GR, de Wit M, Falzon L, Landewé R (2020) Efficacy of pharmacological treatment in rheumatoid arthritis: a systematic literature research informing the 2019 update of the EULAR recommendations for management of rheumatoid arthritis. Ann Rheum Dis 79(6):744–759
Kay J, Upchurch KS (2012) ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford). 51(Suppl 6):5–9
Kellgren JH, Lawrence JS (1957) Radiological assessment of osteo-arthrosis[J]. Ann Rheum Dis 16(4):494–502
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association[J]. Arthritis Rheumatol 29(8):1039–1049
van Riel PLCM, Renskers L (2016) The Disease Activity Score (DAS) and the disease activity score using 28 joint counts (DAS28) in the management of rheumatoid arthritis [J]. Clin Exp Rheumatol 34(5 Suppl 101):S40
Johnson TM, Michaud K, England BR (2020) Measures of rheumatoid arthritis disease activity[J]. Arthritis Care Res 72(10):4–26
Bemani P, Eklund KK, Ali-Hassanzadeh M et al (2021) Proportion of T follicular helper cells in peripheral blood of rheumatoid arthritis patients: a systematic review and meta-analysis[J]. Expert Rev Clin Immunol 17(6):1–14
Duan L, Liu D, Chen H et al (2021) Follicular dendritic cells restrict interleukin-4 availability in germinal centers and foster memory B cell generation[J]. Immunity 54(10):2256-2272.e6
Akiyama M, Yasuok H, Yamaoka K et al (2016) Enhanced IgG4 production by follicular helper 2 T cells and the involvement of follicular helper 1 T cells in the pathogenesis of IgG4-related disease[J]. Arthritis Res Ther 18:167
Zhang F, Wei K, Slowikowski K et al (2019) Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry[J]. Nat Immunol 20(7):1–15
Rao DA, Gurish MF, Marshall JL et al (2017) Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis[J]. Nature 542(7639):110–114
Paula FG, Laura N, Alejandro V et al (2019) Two populations of circulating PD-1hiCD4 T cells with distinct B cell helping capacity are elevated in early rheumatoid arthritis[J]. Rheumatology (Oxford, England) 58(9):1662–1673
Rao DA, Gurish MF, Marshall JL et al (2017) Pathologically expanded peripheral T helper cell subset drives B cells in rheumatoid arthritis[J]. Nature 542(7639):110–114
Zhang LL, Xiao H, Zhang F et al (2021) BAFF, involved in B cell activation through the NF-κB pathway, is related to disease activity and bone destruction in rheumatoid arthritis[J]. Acta Pharmacol Sin 42(10):1665–1675
Aldridge J, Andersson K, Gjertsson I et al (2021) Blood PD-1+TFh and CTLA-4+CD4+ T cells predict remission after CTLA-4Ig treatment in early rheumatoid arthritis[J]. Rheumatology (Oxford) 61(3):1233–1242
Taniguchi T, Takata M, Ikeda A et al (1997) Failure of germinal center formation and impairment of response to endotoxin in tumor necrosis factor alpha-deficient mice[J]. Lab Investig 77(6):647–658
Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A (2016) Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Res Ther 22(18):93
Ueno H (2016) T follicular helper cells in human autoimmunity. Curr Opin Immunol 43:24–31
Zhang Y, Li Y, Lv TT et al (2015) Elevated circulating Th17 and follicular helper CD4+ T cells in patients with rheumatoid arthritis[J]. APMIS 123(8):659–666
Funding
This work was supported by a grant from the National Natural Youth Foundation of China (No.81901662).
Author information
Authors and Affiliations
Contributions
Shaowei Pan and Xiaoli Zhang wrote the main manuscript text. Jiaomei Cheng, Yuzi Tian, and Shuangyun Tan collected the patient’s samples. Xiaoyu Xiao and Huali Zhang participated in the revision of the main manuscript text. Junyu Zhou prepared Figs. 3–5. Tong Li and Shiyao Wu guided the revision of the article and sample size calculation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Shaowei Pan and Xiaoyu Xiao contributed equally.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pan, S., Xiao, X., Li, T. et al. Definition of follicular helper T cell and cytokines expression in synovial fluid of rheumatoid arthritis. Clin Rheumatol 43, 129–135 (2024). https://doi.org/10.1007/s10067-023-06772-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-023-06772-9