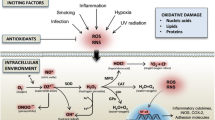
Abstract
This study was performed to evaluate the effects of the TNF-α inhibitor etanercept on oxidation stress markers representing DNA damage, lipid peroxidation, and protein glycosylation. Twenty-two rheumatoid arthritis (RA) patients underwent etanercept treatment. The levels of serum total, urinary total, and urinary free pentosidine, which is an advanced glycation end-product (AGE), of urinary Nε-hexanoyl lysine (Nε-HEL), and of 8-hydroxy-deoxy guanosine (8-OHdG) were measured at baseline and at 3 and 6 months after the initial treatment with etanercept. Serum total and urinary total pentosidine levels were reduced at 6 months after the initial treatment with etanercept, and urinary free pentosidine levels were reduced at 3 and 6 months. Urinary Nε-HEL levels were also reduced at 3 and 6 months, and urinary 8-OHdG levels were reduced at 6 months. Serum total and urinary total pentosidine levels in RA patients correlated with the number of swelling joints and tender joints, and urinary total pentosidine levels correlated with the Disease Activity Score using 28 joints (DAS28). This study demonstrated that etanercept acts as a regulator against pentosidine formation, oxidative DNA damage, and lipid peroxidation in RA patients.
Similar content being viewed by others
References
Hitchon CA, El-Gabalawy HS (2004) Oxidation in rheumatoid arthritis. Arthritis Res Ther 6:265–278
Halliwell B (1995) Oxygen radicals, nitric oxide and human inflammatory joint disease. Ann Rheum Dis 54:505–510
Biemond P, Swaak AJ, Koster JF (1984) Protective factors against oxygen free radicals and hydrogen peroxide in rheumatoid arthritis synovial fluid. Arthritis Rheum 27:760–765
Woo CH, Kim TH, Choi JA, et al (2006) Inhibition of receptor internalization attenuates the TNF alpha-induced ROS generation in non-phagocytic cells. Biochem Biophys Res Commun 351:972–978
Sakon S, Xue X, Takekawa M et al (2003) NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J 22:3898–3909
Takahashi M, Ohishi T, Aoshima H, Kawana K, Kushida K, Inoue T, Horiuchi K (1993) The Maillard protein cross-link pentosidine in urine from diabetic patients. Diabetologia 36:664–667
Jikimoto T, Nishikubo Y, Koshiba M, et al (2002) Thioredoxin as a biomarker for oxidative stress in patients with rheumatoid arthritis. Mol Immunol 38:765–772
Mitoma H, Horiuchi T, Hatta N, Tsukamoto H, Harashima S, Kikuchi Y, Otsuka J, Okamura S, Fujita S, Harada M (2005) Infliximab induces potent anti-inflammatory responses by outside-to-inside signals through transmembrane TNF-alpha. Gastroenterology 128:376–392
Van den Brande JM, Braat H, van den Brink GR, Versteeg HH, Bauer CA, Hoedemaeker I, van Montfrans C, Hommes DW, Peppelenbosch MP, van Deventer SJ (2003) Infliximab but not etanercept induces apoptosis in lamina propria T-lymphocytes from patients with Crohn’s disease. Gastroenterology 124:1774–1785
Lugering A, Schmidt M, Lugering N, Pauels HG, Domschke W, Kucharzik T (2001) Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 121:1145–1157
Rall LC, Roubenoff R, Meydani SN, Han SN, Meydani M (2000) Urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J Nutr Biochem 11:581–584
Jaswal S, Mehta HC, Sood AK, Kaur J (2003) Antioxidant status in rheumatoid arthritis and role of antioxidant therapy. Clin Chim Acta 338:123–129
Kato Y, Mori Y, Makino Y, Morimitsu Y, Hiroi S, Ishikawa T, Osawa T (1999) Formation of Nepsilon-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem 274:20406–20414
Winrow VR, Winyard PG, Morris CJ, Blake DR (1993) Free radicals in inflammation: second messengers and mediators of tissue destruction. Br Med Bull 49:506–522
Mapp PI, Grootveld MC, Blake DR (1995) Hypoxia, oxidative stress and rheumatoid arthritis. Br Med Bull 51:419–436
Kumar DA, Raju KV, Settu K, Kumanan K, Puvanakrishnan R (2006) Effect of a derivatized tetrapeptide from lactoferrin on nitric oxide mediated matrix metalloproteinase-2 production by synovial fibroblasts in collagen-induced arthritis in rats. Peptides 27:1434–1442
Bates EJ, Johnson CC, Lowther DA (1985) Inhibition of proteoglycan synthesis by hydrogen peroxide in cultured bovine articular cartilage. Biochim Biophys Acta 838:221–228
Baker MS, Bolis S, Lowther DA (1991) Oxidation of articular cartilage glyceraldehyde-3-phosphate dehydrogenase (G3PDH) occurs in vivo during carrageenin-induced arthritis. Agents Actions 32:299–304
Schalkwijk J, van den Berg WB, van de Putte LB, Joosten LA (1986) An experimental model for hydrogen peroxide-induced tissue damage. Effects of a single inflammatory mediator on (peri)articular tissues. Arthritis Rheum 29:532–538
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Invest 85:632–639
Bax BE, Alam AS, Banerji B et al (1992) Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem Biophys Res Commun 183:1153–1158
Bonizzi G, Piette J, Merville MP, Bours V (2000) Cell type-specific role for reactive oxygen species in nuclear factor-κB activation by interleukin-1. Biochem Pharmacol 59:7–11
Bonizzi G, Piette J, Schoonbroodt S, Greimers R, Havard L, Merville MP, Bours V (1999) Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol 19:1950–1960
Jacquier-Sarlin MR, Jornot L, Polla BS (1995) Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. J Biol Chem 270:14094–14099
Sung JY, Hong JH, Kang HS, Choi I, Lim SD, Lee JK, Seok JH, Lee JH, Hur GM (2000) Methotrexate suppresses the interleukin-6 induced generation of reactive oxygen species in the synoviocytes of rheumatoid arthritis. Immunopharmacology 47:35–44
Miesel R, Murphy MP, Kroger H (1996) Enhanced mitochondrial radical production in patients which rheumatoid arthritis correlates with elevated levels of tumor necrosis factor alpha in plasma. Free Radic Res 25:161–169
Mur E, Zabernigg A, Hilbe W, Eisterer W, Halder W, Thaler J (1997) Oxidative burst of neutrophils in patients with rheumatoid arthritis: influence of various cytokines and medication. Clin Exp Rheumatol 15:233–237
den Broedr AA (2003) Neutrophil migration and production of reactive oxygen species during treatment with a fully human anti-tumor necrosis factor-alpha monoclonal antibody in patients with rheumatoid arthritis. J Rheumatol 30:232–237
Kirchner S, Holler E, Haffner S, Andreesen R, Eissner G (2004) Effect of different tumor necrosis factor (TNF) reactive agents on reverse signaling of membrane integrated TNF in monocytes. Cytokine 28:67–74
Sieper J, Van Den Brande J (2005) Diverse effects of infliximab and etanercept on T lymphocytes. Semin Arthritis Rheum 34(Suppl1):23–27
Halliwell B, Hoult JR, Blake DR (1988) Oxidants, inflammation, and anti-inflammatory drugs. FASEB J 2:2867–2873
Takahashi M (2006) Pentosidine, an advanced glycation endproduct, and arthritis. Curr Rheumatol Rev 2:319–324
Erhola M, Toyokuni S, Okada K, Tanaka T, Hiai H, Ochi H, Uchida K, Osawa T, Nieminen MM, Alho H, Kellokumpu-Lehtinen P (1997) Biomarker evidence of DNA oxidation in lung cancer patients: association of urinary 8-hydroxy-2’-deoxyguanosine excretion with radiotherapy, chemotherapy, and response to treatment. FEBS Lett 409:287–291
Rodriguez-Garcia J, Requena JR, Rodriguez-Segade S (1998) Increased concentrations of serum pentosidine in rheumatoid arthritis. Clin Chem 44:250–255
Takahashi M, Kushida K, Ohishi T et al (1994) Quantitative analysis of crosslinks pyridinoline and pentosidine in articular cartilage of patients with bone and joint disorders. Arthritis Rheum 37:724–728
Verzijl N, DeGroot J, Bank RA, Bayliss MT, Bijlsma JW, Lafeber FP, Maroudas A, TeKoppele JM. (2001) Age-related accumulation of the advanced glycation endproduct pentosidine in human articular cartilage aggrecan: the use of pentosidine levels as a quantitative measure of protein turnover. Matrix Biol 20:409–417
Takahashi M, Suzuki M, Kushida K, Miyamoto S, Inoue T (1997) Relationship between pentosidine levels in serum and urine and activity in rheumatoid arthritis. Br J Rheumatol 36:637–642
Schraufstatter IU, Hyslop PA, Jackson JH, Cochrane CG (1988) Oxidant-induced DNA damage of target cells. J Clin Invest 82:1040–1050
Kasai H, Crain PF, Kuchino Y, Nishimura S, Ootsuyama A, Tanooka H (1986) Formation of 8-hydroxyguanine moiety in cellular DNA by agents producing oxygen radicals and evidence for its repair. Carcinogenesis (Lond) 7:1849–1851
Toyokuni S (1999) Reactive oxygen species-induced molecular damage and its application in pathology. Pathol Int 49:91–102
Honda M, Yamada Y, Tomonaga M, Ichinose H, Kamihira S (2000) Correlation of urinary 8-hydroxy-2’-deoxyguanosine (8-OHdG), a biomarker of oxidative DNA damage, and clinical features of hematological disorders: a pilot study. Leuk Res 24:461–468
Kim JY, Mukherjee S, Ngo LC, Christiani DC (2004) Urinary 8-hydroxy-2’-deoxyguanosine as a biomarker of oxidative DNA damage in workers exposed to fine particulates. Environ Health Perspect 112:666–671
Kato Y, Mori Y, Makino Y, Morimitsu Y, Hiroi S, Ishikawa T, Osawa T (1999) Formation of Nepsilon-(hexanonyl)lysine in protein exposed to lipid hydroperoxide. A plausible marker for lipid hydroperoxide-derived protein modification. J Biol Chem 274:20406–20414
Kato Y, Yoshida A, Naito M, Kawai Y, Tsuji K, Kitamura M, Kitamoto N, Osawa T (2004) Identification and quantification of N(epsilon)-(Hexanoyl)lysine in human urine by liquid chromatography/tandem mass spectrometry. Free Radic Biol Med 37:1864–1874
Thannickal VJ, Fanburg BL (2000) Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 279:1005–1028
Thabrew MI, Senaratna L, Samarawickrema N, Munasinghe C (2001) Antioxidant potential of two polyherbal preparations used in Ayurveda for the treatment of rheumatoid arthritis. J Ethnopharmacol 76:285–291
Halliwell B (1994) Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet 344:721–724
Bae SC, Kim SJ, Sung MK (2003) Inadequate antioxidant nutrient intake and altered plasma antioxidant status of rheumatoid arthritis patients. J Am Coll Nutr 22:311–315
Kamanli A, Naziroglu M, Aydilek N, Hacievliyagil C (2004) Plasma lipid peroxidation and antioxidant levels in patients with rheumatoid arthritis. Cell Biochem Funct 22:53–57
Araujo V, Arnal C, Boronat M, Ruiz E, Dominguez C (1998 ) Oxidant-antioxidant imbalance in blood of children with juvenile rheumatoid arthritis. Biofactors 8:155–159
Karatas F, Ozates I, Canatan H, Halifeoglu I, Karatepe M, Colakt R (2003) Antioxidant status & lipid peroxidation in patients with rheumatoid arthritis. Indian J Med Res 118:178–181
Cao G, Prior RL (1998) Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin Chem 44:1309–1325
Wang CC, Chu CY, Chu KO, Choy KW, Khaw KS, Rogers MS, Pang CP (2004) Trolox-equivalent antioxidant capacity assay versus oxygen radical absorbance capacity assay in plasma. Clin Chem 50:952–954
Schofield D, Braganza JM (1996) Shortcomings of an automated assay for total antioxidant status in biological fluids. Clin Chem 42:1712–1714
Lamont J, Campbell J, FitzGerald P. (1997) Measurement of individual vs total antioxidants. Clin Chem 43:852–854
Acknowledgments
In this study, I wish to express my gratitude to the offer of patient’s data for the orthopedics clinic of Shimazu (Sizuoka, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kageyama, Y., Takahashi, M., Nagafusa, T. et al. Etanercept reduces the oxidative stress marker levels in patients with rheumatoid arthritis. Rheumatol Int 28, 245–251 (2008). https://doi.org/10.1007/s00296-007-0419-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-007-0419-1