Abstract
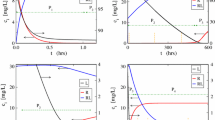
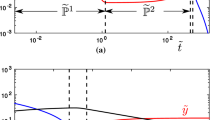
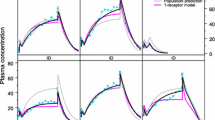
The multi-scale dynamics of a two-compartment with first order absorption Target-Mediated Drug Disposition (TMDD) pharmacokinetics model is analysed, using the Computational Singular Perturbation (CSP) algorithm. It is shown that the process evolves along two Slow Invariant Manifolds (SIMs), on which the most intense components of the model are equilibrated, so that the less intensive are the driving ones. The CSP tools allow for the identification of the components of the TMDD model that (i) constrain the evolution of the process on the SIMs, (ii) drive the system along the SIMs and (iii) generate the fast time scales. Among others, such diagnostics identify (i) the factors that determine the start and the duration of the period in which the ligand-receptor complex acts and (ii) the processes that determine its degradation rate. The counterintuitive influence of the process that transfers the ligand from the tissue to the main compartment, as it is manifested during the final stage of the process, is studied in detail.













Similar content being viewed by others
References
Abraham AK, Krzyzanski W, Mager DE (2007) Partial derivative—based sensitivity analysis of models describing target-mediated drug disposition. AAPS J 9(2):E181–E189
Bakshi S, de Lange E, van der Graaf P, Danhof M, Peletier L (2016) Understanding the behavior of systems pharmacology models using mathematical analysis of differential equations: prolactin modeling as a case study. CPT Pharmacomet Syst Pharmacol 5(7):339–351
Dada JO, Mendes P (2011) Multi-scale modelling and simulation in systems biology. Integr Biol 3:86–96
Danhof M, de Jongh J, De Lange EC, Della Pasqua O, Ploeger BA, Voskuyl RA (2007) Mechanism-based pharmacokinetic-pharmacodynamic modeling: biophase distribution, receptor theory, and dynamical systems analysis. Annu Rev Pharmacol Toxicol 47:357–400
Diamantis DJ, Mastorakos E, Goussis DA (2015) \(\text{ H }_2/\text{ air }\) autoignition: the nature and interaction of the developing explosive modes. Combust Theor Model 19:382–433
Dostalek M, Gardner I, Gurbaxani BM, Rose RH, Chetty M (2013) Pharmacokinetics, pharmacodynamics and physiologically-based pharmacokinetic modelling of monoclonal antibodies. Clin Pharmacokinet 52(2):83–124
Dua P, Hawkins E, van der Graaf PH (2015) A tutorial on target-mediated drug disposition (TMDD) models. CPT Pharmacomet Syst Pharmacol 4:324–337
Fenichel N (1979) Geometric singular perturbation theory for ordinary differential equations. J Differ Equ 31(1):53–98
Gibiansky L (2016) Personal communication
Gibiansky L, Gibiansky E (2009) Target-mediated drug disposition model: approximations, identifiability of model parameters and applications to the population pharmacokinetic-pharmacodynamic modeling of biologics. Expert Opin Drug Metab Toxicol 5(7):803–812
Gibiansky L, Gibiansky E, Kakkar T, Ma P (2008) Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn 35(5):573–591
Gibiansky L, Gibiansky E (2017) Target-mediated drug disposition model for drugs with two binding sites that bind to a target with one binding site. J Pharmacokinet Pharmacodyn 44(5):463–475
Goussis DA (2012) Quasi steady state and partial equilibrium approximations: their relation and their validity. Combust Theor Model 16(5):869–926
Goussis DA (2013) The role of slow system dynamics in predicting the degeneracy of slow invariant manifolds: the case of vdP relaxation-oscillations. Physica D 248:16–32
Goussis DA (2015) Model reduction: when singular perturbation analysis simplifies to partial equilibrium approximation. Combust Flame 162(4):1009–1018
Goussis DA, Lam SH (1992) A study of homogeneous methanol oxidation kinetics using CSP. Proc Combust Inst 24(1):113–120
Goussis DA, Maas U (2011) Model reduction for combustion chemistry. Fluid Mech Appl 95:193–220
Goussis DA, Najm HN (2006) Model reduction and physical understanding of slowly oscillating processes: the circadian cycle. SIAM Multiscale Model Simul 5:1297–1332
Goussis DA, Skevis G (2005) Nitrogen chemistry controlling steps in methane-air premixed flames. In: Bathe KJ (ed) Computational fluid and solid mechanics. Elsevier, Amsterdam, pp 650–653
Hadjinicolaou M, Goussis DA (1998) Asymptotic solution of stiff pdes with the CSP method: the reaction diffusion equation. SIAM J Sci Comput 20(3):781–810
Hek G (2010) Geometric singular perturbation theory in biological practice. J Math Biol 60(3):347–386
Kaper TJ (1999) An introduction to geometric methods and dynamical systems theory for singular perturbation problems. In: Cronin J, Robert J, O’Malley E (eds) Analyzing multiscale phenomena using singular perturbation methods. Proceedings of symposia in applied mathematics, vol 56. American Mathematical Society, Baltimore, pp 85–131
Kaper HG, Kaper TJ, Zagaris A (2015) Geometry of the computational singular perturbation method. Math Model Nat Phenom 10(3):16–30
Koch G, Jusko WJ, Schropp J (2017) Target-mediated drug disposition with drug-drug interaction, part I: single drug case in alternative formulations. J Pharmacokinet Pharmacodyn 44(1):17–26
Koch G, Jusko WJ, Schropp J (2017) Target mediated drug disposition with drug-drug interaction, part II: competitive and uncompetitive cases. J Pharmacokinet Pharmacodyn 44(1):27–42
Kooshkbaghi M, Frouzakis CE, Boulouchos K, Karlin IV (2015) n-Heptane/air combustion in perfectly stirred reactors: dynamics, bifurcations and dominant reactions at critical conditions. Combust Flame 162(9):3166–3179
Kourdis PD, Goussis DA (2013) Glycolysis in saccharomyces cerevisiae: algorithmic exploration of robustness and origin of oscillations. Math Biosci 243:190–214
Kourdis PD, Steuer R, Goussis DA (2010) Physical understanding of complex multiscale biochemical models via algorithmic simplification: glycolysis in saccharomyces cerevisiae. Physica D 239(18):1798–1817
Kourdis PD, Palasantza AG, Goussis DA (2013) Algorithmic asymptotic analysis of the NF-\(\kappa \)B signalling system. Comput Math Appl 65:1516–1534
Kuehn C (2015) Multiple time scale dynamics. Springer, Berlin
Lam SH, Goussis DA (1988) Understanding complex chemical kinetics with computational singular perturbation. Proc Combust Inst 22:931–941
Lam SH, Goussis DA (1994) CSP method for simplifying kinetics. Int J Chem Kinet 26(4):461–486
Levy G (1994) Pharmacologic target-mediated drug disposition. Clin Pharmacol Ther 56(3):248–252
Lovas T (2012) Model reduction techniques for chemical mechanisms. In: Patel V (ed) Chemical kinetics. InTech, Rijeka, pp 79–114
Lu T, Yoo CS, Chen JH, Law CK (2008) Analysis of a turbulent lifted hydrogen/air jet flame from direct numerical simulation with computational singular perturbation. In: 46th AIAA aerospace sciences meeting and exhibit. Paper AIAA-2008-1013
Ma P (2012) Theoretical considerations of target-mediated drug disposition models: simplifications and approximations. Pharm Res 29(3):866–882
Maas J, Tomlin A (2013) Time-scale splitting-based mechanism reduction. In: Cleaner combustion—green energy and technology. Springer, London, pp 467–484
Mager DE (2006) Target-mediated drug disposition and dynamics. Biochem Pharmacol 72(1):1–10
Mager DE, Jusko WJ (2001) General pharmacokinetic model for drugs exhibiting target-mediated drug disposition. J Pharmacokinet Pharmacodyn 28(6):507–532
Mager DE, Krzyzanski W (2005) Quasi-equilibrium pharmacokinetic model for drugs exhibiting target-mediated drug disposition. Pharm Res 22(10):1589–1596
Maris DT, Goussis DA (2015) The hidden dynamics of the Rössler attractor. Physica D 295–296:66–90
Patsatzis DG, Maris DT, Goussis DA (2016) Asymptotic analysis of a target-mediated drug disposition model: algorithmic and traditional approaches. Bull Math Biol 78(6):1121–1161
Peletier LA, Gabrielsson J (2009) Dynamics of target-mediated drug disposition. Eur J Pharm Sci 38(5):445–464
Peletier LA, Gabrielsson J (2012) Dynamics of target-mediated drug disposition: characteristic profiles and parameter identification. J Pharmacokinet Pharmacodyn 39(5):429–451
Schnell S, Grima R, Maini P (2007) Multiscale modeling in biology. Am Sci 95(2):134–142
Senthamaraikkannan G, Gates I, Prasad V (2016) Modeling, estimation and optimization in coreflooding experiments for coalbed methane production. Chem Eng Sci 141:75–85
Surovtsova I, Simus N, Hübner K, Sahle S, Kummer U (2012) Simplification of biochemical models: a general approach based on the analysis of the impact of individual species and reactions on the systems dynamics. BMC Syst Biol 6(1):14
Turányi T, Tomlin AS (2014) Analysis of kinetic reaction mechanisms. Springer, Berlin
Valorani M, Najm HN, Goussis DA (2003) CSP analysis of a transient flame-vortex interaction: time scales and manifolds. Combust Flame 134(1–2):35–53
Valorani M, Goussis DA, Creta F, Najm HN (2005) Higher order corrections in the approximation of low-dimensional manifolds and the construction of simplified problems with the CSP method. J Comput Phys 209(2):754–786
van der Graaf PH, Benson N, Peletier LA (2016) Topics in mathematical pharmacology. J Dyn Differ Equ 28:1337–1356
Wang L, Han X, Cao Y, Najm HN (2017) Computational singular perturbation analysis of stochastic chemical systems with stiffness. J Comput Phys 335:404–425
Zagaris A, Kaper HG, Kaper TJ (2004) Analysis of the computational singular perturbation reduction method for chemical kinetics. J Nonlinear Sci 14(1):59–91
Zagaris A, Kaper HG, Kaper TJ (2004) Fast and slow dynamics for the computational singular perturbation method. SIAM Multiscale Model Simul 2(4):613–638
Zhang Y, D’Argenio DZ (2016) Feedback control indirect response models. J Pharmacokinet Pharmacodyn 43(4):343–358
Acknowledgements
Many useful discussions with Dr. L. Gibiansky are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Appendices
A Appendix: The CSP basis vectors for the \(\hbox {M}=2\) case
Considering the state vector defined as \(\mathbf {y}=[L_d,~L,~L_t,~R,~RL]^T\), the CSP vectors in the case where \(M=2\) are computed as follows. Since the fast variables are R and RL, a proper initial guess is:
With such a choice it is guaranteed that \(\mathbf {a}_r\) has a component in the fast subspace of the tangent space along \(SIM_2\) (Lam and Goussis 1994); i.e., a component along the axis of the fast variables R and RL. After one \(\mathbf {b}^r\) and one \(\mathbf {a}_r\)-refinements, the following CSP vectors are obtained:
where as mentioned in Sect. 6:
B Appendix: The CSP basis vectors for the \(\hbox {M}=3\) case
Considering the state vector as in “Appendix A”, the CSP vectors in the case where \(M=3\) are computed as follows. Since the variables associated the most to the fast time scales are L, RL and R, a proper initial guess is:
With such a choice it is guaranteed that \(\mathbf {a}_r\) has a component in the fast subspace of the tangent space along \(SIM_3\) (Lam and Goussis 1994); i.e., a component along the axis of the fast variables L, RL and R. After one \(\mathbf {b}^r\) and one \(\mathbf {a}_r\)-refinements, the following CSP vectors are obtained:
where
-
\(S_1=D_{en}(X(k_{el}+k_{pt})+Yk_{deg})^2+k_{pt}k_{tp}(2k_{deg} k_{off}Z k_{int}k_{on}LY)\)
-
\(S_2=k_{deg}k_{off}k_{pt}k_{tp}X\)
-
\(S_3=k_{int}k_{on}k_{pt}k_{tp}LX\)
-
\(S_4=D_{en}Xk_{pt}(X(k_{el}+k_{pt})+Yk_{deg})\)
-
\(S_5=k_{deg}k_{off}k_{pt}(X(k_{el}+k_{pt})+Yk_{deg})\)
-
\(S_6=k_{int}k_{on}k_{pt}L(X(k_{el}+k_{pt})+Yk_{deg})\)
-
\(S_7=D_{en}XYk_{pt}k_{tp}\)
-
\(S_8=k_{deg}k_{off}k_{pt}k_{tp}Y\)
-
\(S_9=D_{en}(X(k_{el}+k_{pt})+k_{deg}Y)^2+k_{pt}k_{tp}(X^2D_{en} +k_{deg}k_{off}Z)\)
-
\(S_{10}=D_{en}XZk_{pt}k_{tp}\)
-
\(S_{11}=D_{en}((k_{el}+k_{pt})X+Yk_{deg})^2+k_{pt}k_{tp}(D_{en}X^2 +k_{int}k_{on}LY)\qquad +2k_{deg}^2(k_{el}+k_{pt})(k_{off}+k_{int})Y\)
-
\(S_{12}=k_{pt}k_{tp}k_{deg}k_{on}LY\)
-
\(S=D_{en}(X(k_{el}+k_{pt}+Yk_{deg})^2+k_{pt}k_{tp}(X^2D_{en} +k_{deg}k_{off}Z)\)
where
-
\(H_1=D_{en}^2+k_{pt}k_{tp}X_1\)
-
\(H_2=k_ak_{pt}X_1\)
-
\(H_3=k_{pt}XD_{en}^2(X(k_{el}+k_{pt}+k_{int}Z)\)
-
\(H_4=((k_{el}+k_{pt})XD_{en}+k_{int}ZD_{en})^2\)
-
\(H_5=k_{pt}k_{int} k_{on} L ((k_{el}+k_{pt}) XD_{en}+k_{int}ZD_{en})\)
-
\(H_6=k_{pt}k_{deg} k_{off} ((k_{el}+k_{pt})XD_{en} +k_{int}ZD_{en})\)
Rights and permissions
About this article
Cite this article
Michalaki, L.I., Goussis, D.A. Asymptotic analysis of a TMDD model: when a reaction contributes to the destruction of its product. J. Math. Biol. 77, 821–855 (2018). https://doi.org/10.1007/s00285-018-1234-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-018-1234-x