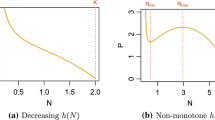
Abstract
Predator interference, that is, a decline in the per predator consumption rate as predator density increases, is generally thought to promote predator–prey stability. Indeed, this has been demonstrated in many theoretical studies on predator–prey dynamics. In virtually all of these studies, the stabilization role is demonstrated as a weakening of the paradox of enrichment. With predator interference, stable limit cycles that appear as a result of environmental enrichment occur for higher values of the environmental carrying capacity of prey, and even a complete absence of the limit cycles can happen. Here we study predator–prey dynamics using the Rosenzweig–MacArthur-like model in which the Holling type II functional response has been replaced by a predator-dependent family which generalizes many of the commonly used descriptions of predator interference. By means of a bifurcation analysis we show that sufficiently strong predator interference may bring about another stabilizing mechanism. In particular, hysteresis combined with (dis)appearance of stable limit cycles imply abrupt increases in both the prey and predator densities and enhanced persistence and resilience of the predator–prey system. We encourage refitting the previously collected data on predator consumption rates as well as for conducting further predation experiments to see what functional response from the explored family is the most appropriate.








Similar content being viewed by others
References
Abrams PA (1994) The fallacies of “ratio-dependent” predation. Ecology 75:1842–1850
Aguirre P, Flores JD, González-Olivares E (2014) Bifurcations and global dynamics in a predator–prey model with a strong allee effect on the prey, and a ratio-dependent functional response. Nonlinear Anal Real World Appl 16:235–249
Arnqvist G, Jones TM, Elgar MA (2006) Sex-role reversed nuptial feeding reduces male kleptoparasitism of females in Zeus bugs (Heteroptera: Veliidae). Biol Lett 2:491–493
Baer SM, Kooi BW, Kuznetsov YA, Thieme HR (2006) Multiparametric bifurcation analysis of a basic two-stage population model. SIAM J Appl Math 66:1339–1365
Beddington JR (1975) Mutual interference between parasites or predators and its effect on searching efficiency. J Animal Ecol 44:331–340
Begon M, Harper JL, Townsend CR (1990) Ecology: individuals, populations and communities, 2nd edn. Blackwell Scientific Publications, Oxford
Berec L (2010) Impacts of foraging facilitation among predators on predator–prey dynamics. Bull Math Biol 72:94–121
Boukal DS, Sabelis MW, Berec L (2007) How predator functional responses and Allee effects in prey affect the paradox of enrichment and population collapses. Theor Popul Biol 72:136–147
Boukal DS, Berec L, Krivan V (2008) Does sex-selective predation stabilize or destabilize predator–prey dynamics? PLoS One 3(7):e2687
Briggs CJ, Hoopes MF (2004) Stabilizing effects in spatial parasitoid–host and predator–prey models: a review. Theor Popul Biol 65:299–315
Cantrell RS, Cosner C (2001) On the dynamics of predator-prey models with the Beddington–DeAngelis functional response. J Math Anal Appl 257:206–222
Cosner C, DeAngelis D, Ault JS, Olson DB (1999) Effects of spatial grouping on the functional response of predators. Theor Popul Biol 56:65–75
DeAngelis DL, Goldstein RA, O’Neill RV (1975) A model for tropic interaction. Ecology 56:881–892
Fryxell JM, Mosser A, Sinclair ARE, Packer C (2007) Group formation stabilizes predator–prey dynamics. Nature 449:1041–1044
Gascoigne JC, Lipcius RN (2004) Allee effects driven by predation. J Appl Ecol 41:801–810
Gross T, Ebenhöh W, Feudel U (2004) Enrichment and foodchain stability: the impact of different forms of predator–prey interaction. J Theor Biol 227:349–358
Hassell MP, Lawton JH, Beddington JR (1976) The components of arthropod predation. 1. The prey death rate. J Animal Ecol 45:135–164
Hilker FM, Langlais M, Malchow H (2009) The Allee effect and infectious diseases: extinction, multistability, and the (dis-)appearance of oscillations. Am Nat 173:72–88
Holling CS (1959) Some characteristics of simple types of predation and parasitism. Can Entomol 91:385–398
Holt RD (2011) Natural enemy–victim interactions: do we have a unified theory yet? In: Scheiner SM, Willig MR (eds) The theory of ecology. University of Chicago Press, Chicago, pp 125–162
Huisman G, DeBoer RJ (1997) A formal derivation of the “Beddington” functional response. J Theor Biol 185:389–400
Kohlmeier C, Ebenhöh W (1995) The stabilizing role of cannibalism in a predator–prey system. Bull Math Biol 57:401–411
Kot M (2001) Elements of mathematical ecology. Cambridge University Press, Cambridge
Kuznetsov YA (1998) Elements of applied bifucation theory, 2nd edn. Applied Mathematical Sciences, vol 112. Springer, Berlin
Křivan V, Vrkoč I (2004) Should “handled” prey be considered? Some consequences for functional response, predator–prey dynamics and optimal foraging theory. J Theor Biol 227:167–174
Lade SJ, Tavoni A, Levin SA, Schlüter M (2013) Regime shifts in a social-ecological system. Theor Ecol 6:359–372
Lotka AJ (1920) Undamped oscillations derived from the law of mass action. J Am Chem Soc 42:1595–1599
Ludwig D, Jones DD, Holling CS (1978) Qualitative analysis of insect outbreak systems: the spruce budworm and forest. J Animal Ecol 47:315–332
Magnússon KG (1999) Destabilizing effect of cannibalism on a structured predator–prey system. Math Biosci 155:61–75
Murdoch WW, Oaten A (1975) Predation and population stability. In: Macfayden A (ed) Advances in ecological research, vol 9. Academic Press, London, pp 1–131
Oaten A, Murdoch WM (1975) Functional response and stability in predator–prey systems. Am Nat 109:289–298
Pavlova V, Berec L, Boukal DS (2010) Caught between two Allee effects: trade-off between reproduction and predation risk. J Theor Biol 264:787–798
Rogers DJ, Hassell MP (1974) General models for insect parasite and predator searching behaviour: interference. J Animal Ecol 43:239–253
Rosenzweig ML (1971) Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science 171:385–387
Rosenzweig ML, MacArthur RH (1963) Graphical representation and stability conditions of predator–prey interactions. Am Nat 97:209–223
Ruxton GD (1995) Short term refuge use and stability of predator–prey models. Theor Popul Biol 47:1–17
Ruxton GD, Gurney WSC, Roos AMD (1992) Interference and generation cycles. Theor Popul Biol 42:235–253
Scheffer M, Carpenter SR (2003) Catastrophic regime shifts in ecosystems: linking theory to observation. Trends Ecol Evol 18:648–656
Scheffer M, Carpenter SR, Foley JA, Folke C, Walker B (2001) Catastrophic shifts in ecosystems. Nature 413:591–596
Seydel R (2010) Practical bifurcation and stability analysis, 3r edn. Springer, Berlin
Skalski GT, Gilliam JF (2001) Functional responses with predator interference: viable alternatives to the Holling type II model. Ecology 82:3083–3092
Spradbery JP (1970) Host finding by Rhyssa persuasoria (L.), an ichneumonid parasite of siricid woodwasps. Animal Behav 18:103–114
Volterra V (1926) Fluctuation in the abundance of a species considered mathematically. Nature 118:558–560
van Voorn GAK, Stiefs D, Gross T, Kooi BW, Feudel U, Kooijman SALM (2008) Stabilization due to predator interference: comparison of different analysis approaches. Math Biosci Eng 5:567–583
Vos M, Kooi BW, DeAngelis DL, Mooij WM (2004) Inducible defences and the paradox of enrichment. Oikos 105:471–480
Acknowledgments
LP acknowledges support from the project CZ.1.07/2.2.00/15.0203 “Univerzitní výuka matematiky v měnícím se světě”. LB acknowledges institutional support RVO:60077344.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix A
Appendix A
1.1 A.1 Fold bifurcation
Here we show that if a coexistence equilibrium of the model (1) exists then the condition (10) is equivalent to the existence of one zero eigenvalue of the Jacobian of this model evaluated at this equilibrium. Let us define a function \(g(N,P)\) such that \(f(N,P) = N g(N,P)\) where \(f(N,P)\) is the predator functional response (2). In addition, let \(A\) denote the Jacobian of the model (1) and \([N^*,P^*]\) any coexistence equilibrium of this model. By direct computation we have
where \(g=g(N^*,P^*)\), \(g_N=\partial g(N^*,P^*)/\partial N\) and \(g_P=\partial g(N^*,P^*)/\partial P\). Since for the coexistence equilibrium \(eN^*g=m\), we get
and
Since the functional response (2) implies \(g(N,P) = \lambda (P)/[1+h\lambda (P)N]\), we have
Substituting (27) and (6) into (26), we get
Consequently, the condition (10) is equivalent to the condition \(\det A = 0\) at a coexistence equilibrium \([N^*,P^*]\) and is a necessary condition for this equilibrium to be a limit point. Generic change in a single model parameter will change sign of the determinant \(\det A\). Consequently, the local phase space changes qualitatively.
1.2 A.2 Hopf bifurcation
For a Hopf bifurcation to occur at a parameter value in a two-dimensional system, real parts of the complex eigenvalues of the Jacobian evaluated at the respective system equilibrium have to transversally cross zero (i.e. change sign) when the parameter crosses that value. Denoting by \(a\) the bifurcation parameter and by \(\mu (a) \pm i \omega (a)\) the respective pair of complex eigenvalues, we require \(\mu (a_c)=0\), \(\omega (a_c)>0\), and \(\mu '(a_c) \ne 0\) at a bifurcation point \(a=a_c\) as a necessary conditions for a Hopf bifurcation to occur. we note that
where \(A(a)\) denotes the Jacobian evaluated at the system equilibrium corresponding to the parameter \(a\).
Now we will prove that if a coexistence equilibrium of the model (1) exists then the condition (15) is necessary and sufficient for vanishing of the trace of the Jacobian of the model (1) at that equilibrium. Denoting \(g(N,P)=f(N,P)/N\) then at the coexistence equilibrium
Consequently, at this equilibrium the trace (25) of the Jacobian of the model (1) simplifies to
Since \(r>0\) and according to (6) \(\tfrac{N^*}{K} = \tfrac{C_2}{\varLambda } > 0\) at the coexistence equilibrium, we have
where \(C_2=\tfrac{m}{K(e-hm)}\). Since the model (1) implies \(g=r\lambda /C_1\), a necessary and sufficient condition for vanishing of the trace of the Jacobian of the model (1) at the coexistence equilibrium is
or
which is equivalent to the condition (15).
Similar manipulation with the expression (28) and replacement of \(\varLambda '\) with the right-hand side of (15) gives the second necessary condition
This second condition \(\det A > 0\) excludes the neutral saddle case (with two real opposite eigenvalues).
1.3 A.3 Hopf bifurcation in the special case of \(\lambda (P)\) satisfying (4)
For the special case of \(\lambda (P)\) satisfying (4), the condition (15) simplifies to (17). We give here some properties of the polynomial \(\varphi (\varLambda )=A\varLambda ^3+B\varLambda ^2+C\varLambda +D\) on the left-hand side of (17).
For a coexistence equilibrium to exist, we must have \(e-hm>0\). Consequently, \(A=K^2hb(e-hm)^3>0\) and \(D=erm(e+hm)>0\). The cubic polynomial \(\varphi (\varLambda )\) then satisfies \(\varphi (0)>0\) and \(\lim _{\varLambda \rightarrow \infty } \varphi (\varLambda )=\infty \). Let \(w_B=\frac{hKer-b(e+hm)}{Ke(e-hm)}\) be the value of \(w\) at which \(B=0\). Analogously, let \(w_C=\frac{r(2hm+e)}{m(e-hm)}\) be the value of \(w\) at which \(C=0\). Since
at the coexistence equilibrium, \(B\) decreases linearly with \(w\) and \(C\) increases linearly with \(w\), \(B\) or \(C\) need to be negative for any \(w\) at the coexistence equilibrium. (The proof is evident: let us suppose that \(B \ge 0\) and \(C \ge 0\) concurrently, consequently \(w \le w_B\) and \(w \ge w_C\) which is the contrary to \(w_B<w_C\).)
Let \(\varLambda _{min} = -\frac{B}{3A} + \frac{\sqrt{B^2-3AC}}{3A}\) denote the local minimum of the cubic polynomial \(\varphi (\varLambda )\). Now we will show that this local minimum indeed exists, i.e.} that the discriminant \(d(w)=B^2-3AC\) is positive for any \(w\) at the coexistence equilibrium. The function \(d(w)\) is a quadratic convex function of \(w\) with minimum in \(w_{min}=\frac{bhm+2hKer-2be}{2Ke(e-hm)}\). From this,
Consequently \(\varLambda _{min}\) exists and for \(B<0\) it has to be positive. If \(B\ge 0\), then \(C<0\) and consequently \(\sqrt{B^2-3AC}>B\) also gives positivity of \(\varLambda _{min}\). Simulations show that \(\varphi (\varLambda _{min})\le 0\) and hence that the polynomial (17) has a positive root for any \(w\), but we don’t have any analytical proof for \(\varphi (\varLambda _{min})\le 0\) yet.
1.4 A.4 Bogdanov–Takens bifurcation can occur for small handling times \(h\)
Let us have the handling time \(h\) small enough so that \(r < \tfrac{e-hm}{h}\). This implies \(A<0\) in (20) and since \(C>0\), there exists one positive real root \(\varLambda _c\) of the quadratic equation (19). We will show that this \(\varLambda _c\) satisfies the condition (8) necessary for the corresponding \(P^*\) to be a coexistence equilibrium. Let \(0 < \varLambda _c \le C_2=\tfrac{m}{K(e-hm)}\). Consequently,
which cannot happen and thus the contrary \(\varLambda _c>C_2\) is true. Consequently, the critical Bogdanov–Takens bifurcation point occurs at the coexistence equilibrium \([N^*, P^*]\) that satisfies \(P^*=\lambda ^{-1}(\varLambda _c)\) and \(N^*=\tfrac{m}{(e-hm)\varLambda _c}\). Generic change of any two of the parameters \(\lambda _0, \, K, \, e, \, h, \, m\), and \(r\) will change sign of the trace and determinant of the Jacobian at the corresponding coexistence equilibrium and violate the existence of two zero eigenvalues. This change then necessarily causes a saddle separatrix loop split near the critical parameters.
Rights and permissions
About this article
Cite this article
Přibylová, L., Berec, L. Predator interference and stability of predator–prey dynamics. J. Math. Biol. 71, 301–323 (2015). https://doi.org/10.1007/s00285-014-0820-9
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-014-0820-9