Abstract
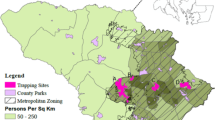
Chronic wasting disease (CWD) is an infectious prion disease that affects mule deer, along with other Cervids. It is a slow-developing, fatal disease which is rare in the free-ranging deer population of Utah. We present a sex-structured, spatial model for the spread of CWD over heterogeneous landscapes, incorporating both horizontal and environmental transmission pathways. To connect the local movement of deer to the regional spread of CWD, we use ecological diffusion with motility coefficients estimated from mule deer movement data. Ecological diffusion allows for aggregation of populations in desirable habitats and therefore allows for an interaction between density dependent disease transmission and landscape structure. The major innovation presented is use of homogenization to accelerate simulations of disease spread in southeastern Utah, from the La Sal Mountains near Moab to the Abajo Mountains near Monticello. The homogenized model provides accuracy while maintaining fidelity to small-scale habitat effects on deer distribution, including differential aggregation in land cover types with high residence times, with errors comparable to the order parameter measuring separation of small and large scales (\(\epsilon \approx .01\) in this case). We use the averaged coefficients from the homogenized model to explore asymptotic invasion speed and the impact of current population size on disease spread in southeastern Utah.










Similar content being viewed by others
References
Almberg ES, Cross PC, Johnson CJ, Helsey DM (2011) Modeling routes of chronic wasting disease transmission: environmental prion persistence promotes deer popoulation decline and extinction. PLoS ONE 6:e19,896
Anderson RM, May RM (1979) Population biology of infectious diseases. Part 1. Nature 280:361–367
Aronson DG, Weinberger HF (1978) Multidimensional nonlinear diffusion arising in populaiton dynamics. Adv Math 30:33–76
Baeten LA, Powers BE, Jewell JE, Spraker TR, Miller MW (2007) A natural case of chronic wasting disease in a free-ranging moose (Alces alces shirasi). J Wildl Dis 43:309–314
Bar-David S, Lloyd-Smith JO, Getz WM (2006) Dynamics and management of infectious disease in colonizing populations. Ecology 87:1215–1224
Conner MM, Miller MW (2004) Movement patterns and spatial epidemiology of a prion disease in mule deer popoulation units. Ecol Appl 14:1870–1881
Department of Interior GS (2006) The national map LANDFIRE: LANDFIRE national existing vegetation type layer. http://gisdata.usgs.net/website/landfire/
Dewhirst S, Lutscher F (2009) Dispersal in heterogeneous habitats: Thresholds, spatial scales, and approximate rates of spread. Ecology 90:1338–1345
Farnsworth ML, Wolfe LL, Hobbs NT, Burnham KP, Williams ES, Theobald DM, Conner MM, Miller MW (2005) Human land use influences chronic wasting disease prevalence in mule deer. Ecol Appl 15:119–126
Farnsworth ML, Hoeting JA, Hobbs NT, Miller MW (2006) Linking chronic wasting disease to mule deer movement scales: A hierarchical Bayesian approach. Ecol Appl 16:1026–1036
Fitzgibbon WE, Langlais M, Morgan JJ (2001) A mathematical model of the spread of feline leukemia virus (felv) through a highly heterogeneous spatial domain. SIAM J Math Anal 33:570–588
Garlick MJ (2012) Homogenization of large-scale movement models in ecology with application to the spread of chronic wasting disease in mule deer, PhD thesis. Utah State University, Utah
Garlick MJ, Powell JA, Hooten MB, McFarlane LR (2011) Homogenization of large-scale movement models in ecology. Bull Math Biol 73:2088–2108
Gross JE, Miller MW (2001) Chronic wasting disease in mule deer: Disease dynamics and control. J Wildl Manag 65:205–215
Hanks EM, Hooten MB, McFarlane LR, Mock KE (2010) Model-based approaches for characterizing spatial genetic flow. In: JSM proceedings, biometrics section. American Statistical Association
Holmes EE, Lewis MA, Banks JE, Veit RR (1994) Partial differential equations in ecology: spatial interactions and population dynamics. Ecology 75:17–29
Holmes MH (1995) Introduction to perturbation methods. Springer, New York
Hosseini PR, Dhondt AA, Dobson AP (2006) Spatial spread of an emerging infectious disease: conjunctivitis in house finches. Ecology 87:3037–3046
Johns CJ, Mehl CH (2006) A dynamic spatial model for chronic wasting disease in Colorado. J Data Sci 4:21–37
Johnson CJ, Pederson JA, Chappell RJ, McKenzie D, Aiken JM (2006) Oral transmissibility of prion disease is enhanced by binding to soil particles. Public Libr Sci Pathog 3:874–881
Johnson DS, London JM, Lea MA, Durban JW (2008) Continuous-time correlated random walk model for animal telemetry data. Ecology 89:1208–1215
Joly DO, Samuel MD, Langenberg JA, Blanchong JA, Batha CA, Rolley RE, Keane DP, Ribic CA (2006) Spatial epidemiology of chronic wasting disease in Wisconsin white-tailed deer. J Wildl Dis 42:578–588
Kallen A, Arcuri P, Murray JD (1985) A simple model for the spatial spread and control of rabies among foxes. J Theor Biol 116:377–393
Kendall DG (1965) Mathematical models of the spread of infection. Medical Research Council, HMSO, London
Krumm CE, Connor MM, Miller MW (2005) Relative vulnerability of chronic wasting disease infected mule deer to vehicle collisions. J Wildl Dis 41:503–511
Kucera TE (1978) Social behavior and breeding system of the desert mule deer. J Mammal 59:463–476
McFarlane LR (2007) Breeding behavior and space use of male and female mule deer: an examination of potential risk differences for chronic wasting disease infection, Master’s thesis. Utah State University, Utah
Miller MW, Wild MA (2004) Epidemiology of chronic wasting disease in captive white-tailed and mule deer. J Wildl Dis 40:320–327
Miller MW, Williams ES (2003) Prion disease: Horizontal prion transmission in mule deer. Nature 425:35–36
Miller MW, Williams ES, McCarty CW, Spraker TR, Kreeger TJ, Larsen CT, Thorne ET (2000) Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis 36:676–690
Miller MW, Williams ES, Hobbs NT, Wolfe LL (2004) Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10:1003–1006
Miller MW, Hobbs NT, Tavener SJ (2006) Dynamics of prion disease transmission in mule deer. Ecol Appl 16:2208–2214
Miller MW, Swanson HM, Wolfe L, Quartarone FG, Huwer SL, Southwick CH, Lukacs PM (2008) Lions, prions and deer demise. Public Libr Sci ONE 3:1–7
Mitchell AR, Griffiths DF (1980) The finite difference method in partial differential equations. Wiley, New York
Murray JD, Stanley EA, Brown DL (1986) On the spatial spread of rabies among foxes. Proc R Soc Lond B 229:111–150
Nicholson MC, Bowyer RT, Kie JG (1997) Habitat selection and survival of mule deer: tradeoffs associated with migration. J Mammal 78:483–504
Okubo A, Levin SA (2001) Diffusion and ecological problems: modern perspective interdisciplinary applied mathematics. Springer, New York
Okubo A, Maini PK, Williamson MH, Murray JD (1989) On the spatial spread of the grey squirrel in Britain. Proc R Soc Lond Ser B 238:113–125
Ostfeld RS, Glass GE, Keesing F (2005) Spatial epidemiology: an emerging (or re-emerging) discipline. Trends Ecol Evol 20:328–336
Ovaskainen O (2008) Analytical and numerical tools for diffusion-based movement models. Theor Popul Biol 73:198–211
Pech RP, McIlroy JC (1990) A model of the velocity of advamce of foot and mouth disease in feral pigs. J Appl Ecol 27:635–650
Plummer AP, Christensen DR, Monsen SB (1968) Restoring big-game range in Utah, Tech. rep. Utah Division of Fish and Game, Utah
Powell JA (1997) Conditional stability of front solutions. J Math Biol 35:729–747
Powell JA, Zimmermann NE (2004) Multiscale analysis of active seed dispersal contributes to resolving Reid’s paradox. Ecology 85:490–506
Rasmussen R (2012) Southeast utah. http://raysweb.net
Real LA, Biek R (2007) Spatial dynamics and genetics of infectious diseases on heterogeneous landscapes. J R Soc Interface 4:935–948
Schauber EM, Woolf A (2003) Chronic wasting disease in deer and elk: a critique of current models and their application. Wildl Soc Bull 31:610–616
Shigesada N, Kawasaki K (1997) Biological invasions: theory and practice. Oxford University Press, Tokyo
Skellam JG (1951) Random dispersal in theoretical populations. Biometrika 38:196–218
Smith DL, Lucey B, Waller LA, Childs JE, Real LA (2002) Predicting the spatial dynamics of rabies epidemics on heterogeneous landscapes. Proc Natl Acad Sci USA 99:3368–3672
Turchin P (1998) Quantitative analysis of movement. Sinauer Associates, Inc. Publishers, Sunderland
UDWR (2012) Utah division of wildlife resources: chronic wasting disease in Utah. http://wildlife.utah.gov
Watkins BE, Bishop CJ, Bergman EJ, Bronson A, Hale B, Wakeling BF, Carpenter LH, Lutz DW (2007) Habitat guidelines for mule deer: Colorado plateau shrubland and forest ecoregion, Tech. rep. Mule Deer Working Group, Western Association of Fish and Wildlife Agencies
Wild MA, Hobbs NT, Graham MS, Miller MW (2011) The role of predation in disease control: a comparison of selective and nonselective removal on prion disease dynamics in deer. J Wildl Dis 47:78–93
Williams ES (2005) Chronic wasting disease. Vet Pathol 42:530–549
Acknowledgments
This research was partially funded by USGS 1434-06HQRU1555. We would like to thank the Utah Division of Wildlife Resources for providing telemetry data and Ben Crabb for providing landcover data. Any use of trade names is for descriptive purpose only and does not imply endorsement by the U.S. Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garlick, M.J., Powell, J.A., Hooten, M.B. et al. Homogenization, sex, and differential motility predict spread of chronic wasting disease in mule deer in southern Utah. J. Math. Biol. 69, 369–399 (2014). https://doi.org/10.1007/s00285-013-0709-z
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-013-0709-z
Keywords
- Diffusion model
- Ecological diffusion
- Spatial heterogeneity
- Multi-scale modeling
- Asymptotic speed of disease spread