Abstract
Understanding the ecology of the often dense white-tailed deer populations in urban and suburban landscapes is important for mitigating a variety of conflicts that arise with dense human populations, such as issues surrounding zoonotic disease mitigation. We collared white-tailed deer in highly suburban areas of Howard County, Maryland with high-resolution GPS collars. Then, we created autocorrelated kernel density home ranges for broader land use analyses and concurrently used general additive models to characterize fine-scale hourly measures of speed, activity, and proximity to residential buildings. Suburban deer home ranges encompassed approximately 35% direct residential land, and an average of 71 and 129 residential properties were found within female and male core ranges, respectively. Sex, time of day, and day of year all influenced fine-scale speeds, activity levels, and proximity to residential property buildings. Deer moved into residential areas nightly, especially in winter, and exhibited bouts of increased speed and activity shortly after sunrise and sunset, with distinctive seasonal changes. We discuss how variation in home ranges and movements may influence population management success and explore year-round periods of increased risk of deer transporting ticks to residential areas. These findings focus our broad understanding of deer movements in suburban and urban landscapes to improve deer population management and to mitigate the spread of ticks into residential areas.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White-tailed deer (Odocoileus virginianus, hereafter “deer”) are an adaptive species inhabiting rural (Walter et al. 2009), exurban (Storm et al. 2007; Rhoads et al. 2010), suburban, and urban areas (Kilpatrick et al. 2011; Urbanek and Nielsen 2013; Potapov et al. 2014), and are particularly abundant in the suburban landscapes of the eastern United States (Dechen Quinn et al. 2013; Walter et al. 2018). These suburban areas are typically characterized by a network of residential neighborhoods, schools, businesses, and patches of open space or undeveloped land, which provide quality, safe habitat for deer (Potapov et al. 2014). Moreover, fragmented patches of open space, ornamental plantings, and supplemental feeding from suburban residents provide year-round food sources (Grund et al. 2002; Williams and Ward 2006) allowing for and potentially attracting increased deer numbers. These overabundant deer populations have led to conflicts in urban and suburban areas, including increased risk of deer-vehicle collisions (Hussain et al. 2007; DeNicola and Williams 2008), over-browsing of natural and ornamental vegetation (Rooney and Waller 2003), and growing concerns over their role in the prevalence of tick-borne zoonotic diseases (Frank et al. 1998; Walter et al. 2011; Stafford et al. 2017).
Deer in suburban and urban areas exhibit high site fidelity and home ranges are typically smaller than deer in rural areas but still vary between seasons and individuals (Etter et al. 2002; Grund et al. 2002; Porter et al. 2004; Kilpatrick et al. 2011). Deer primarily find cover in undeveloped patches (Potapov et al. 2014), with movement enabled via private properties (residential or business), road rights-of-way, and riparian areas (Kilpatrick and Spohr 2000a, b; Grund et al. 2002). Much of the existing deer research has focused on deer ecology during fall and winter as opposed to studies that would have specific, relevant tick ecology implications. So, there is limited consensus on finer scale deer movements in suburban neighborhoods, with results ranging from slight avoidance to moderate preference for residential properties as foraging areas (Kilpatrick and Spohr 2000a, b; Grund et al. 2002; Storm et al. 2007; Kilpatrick et al. 2011; Potapov et al. 2014). Yet, many studies have documented some minimum level of use of residential areas, with an increase during winter (Kilpatrick and Spohr 2000a, b; Grund et al. 2002; Storm et al. 2007; Kilpatrick et al. 2011; Potapov et al. 2014). Given the needs of wildlife managers grappling with overabundant suburban deer (Urbanek et al. 2011; Stafford and Williams 2017), high quality information regarding fine-scale movements by deer within suburban areas is needed.
While relationships between deer densities, deer space use, and specific tick abundances is highly complex, research is starting to deconstruct the intertwined associations (LoGiudice et al. 2008; Kilpatrick 2014). Interestingly, while Lyme borreliosis or Lyme disease is the most frequently reported tick-borne illness in the eastern U.S., white-tailed deer are incompetent hosts for Borrelia burgdorferi, the bacteria that causes Lyme disease (Telford et al. 1988; Allan et al. 2010). On the other hand, deer are competent reservoirs for pathogens such as Erlichia spp. and Anaplasma phagocytophilum; high deer densities support increases in tick populations; and deer transport ticks through the environment (Telford et al. 1988; Allan et al. 2010; Kilpatrick 2014, Milholland et al. 2021a, b).
Two tick populations of importance in suburban and urban areas of the eastern U.S. include the blacklegged tick (Ixodes scapularis) and lone star tick (Amblyomma americanum; White and Gaff 2018; Milholland et al. 2021a). Both species can transmit a suite of pathogens such as Rickettsia spp. (Rocky Mountain spotted fever), Ehrlichia spp. (human ehrlichiosis), or Borrelia spp. (Lyme disease; Milholland et al. 2021a, b) to humans, and there is mounting evidence regarding their bites causing alpha-gal syndrome (Crispell et al. 2019; Young et al. 2021). Unfortunately, deer can host all parasitic tick stages and are a keystone host for both the blacklegged tick and lone star tick (Kollars et al. 2000; Kilpatrick et al. 2014; Huang et al. 2019; Roden-Reynolds 2020). As the majority of tick bite reports originate from suburban homeowners’ yards (Stafford et al. 2017), movement of deer into residential zones could be contributing to humans encountering ticks, thereby increasing risk of exposure to tick-borne disease (Kilpatrick 2014).
Unfortunately, fine-scale deer movement analysis as it relates to the tick life cycle has not been well studied. Therefore, our objective was to evaluate deer habitat use and movement throughout an urbanized landscape across the annual cycle using high resolution hourly telemetry data. Specifically, we hypothesized that both broad deer habitat uses and fine-scale deer movements as they relate to residential areas would vary across seasons, with variation tied to deer phenology and changes in photoperiod. To assess that hypothesis, we characterized broad measures of suburban land use through annual and seasonal home ranges, and we quantified changes in speed, activity, and distance to individual residential properties using a fine spatial and temporal scale. Then, specific information on deer usage of suburban yards facilitated recommendations for managers wishing to reduce deer and tick populations in specific residential areas, to potentially lower rates of human exposure to tick-borne zoonotic diseases.
Methods
Study area
This study took place in Howard County, Maryland, approximately 29 km south of Baltimore, MD and 43 km north of Washington D.C. Howard County had a human population of approximately 325,690 people and was 650 km2 with an average density of 501 people/km2 (United States Census Bureau 2019). On average, annual rainfall was 1.09 m and annual snowfall was 0.58 m (Kraft 2008). In winter, the average temperature was 0.78 °C, and the average daily minimum temperature was − 4.9 °C. In summer, the average temperature was 22.9 °C, and the average daily maximum temperature was 29.6 °C (Kraft 2008). County forest cover was predominantly oak (Quercus spp.), hickory (Carya spp.), and tulip tree (Liriodendron tulipifera) in the overstory. The understory was often dominated with invasives such as autumn olive (Elaeagnus umbellate), Amur honeysuckle (Lonicera maackii), and multiflora rose (Rosa multiflora). However native species such as Rubus spp., maple (Acer spp.), eastern red cedar (Juniperus virginiana), and black cherry (Prunus serotina) were also common (Kraft 2008).
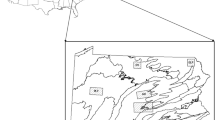
Within Howard County, MD, deer were captured in five Howard County parks including Blandair Regional Park (north section only), Cedar Lane Park, Middle Patuxent Environmental Area, Rockburn Branch Park, and Wincopin Trails System (see Online Resource 1, Table S1 for specific site details). All five parks had some level of recreational use, including sports fields, playgrounds, and walking paths. All five study sites were within the metropolitan boundary of Howard County, which is characterized by greater urban development (Fig. 1). Specifically, within the metropolitan zone designation, human population density increased to 964 persons/km2 from the more rural western portion of the county with 124 persons/km2 (Howard County Department of Planning and Zoning, Research Division 2020).
Map of Howard County, Maryland population density by census tract in persons per square kilometer (2017) and metropolitan zone containing the five county parks selected for deer trapping from 2017–2019. Other county parks are depicted as purple polygons. Individual trapping sites are labeled as A: Middle Patuxent Environmental Area, B: Cedar Lane Park, C: Blandair Regional Park, D: Rockburn Branch Park, and E: Wincopin Trails System/Savage Park
Wildlife managers have used several methods to assess deer densities throughout Howard County, and the available park-specific deer densities are reported in supplemental information Online Resource 1, Table S1. In nearby Delaware, Dion et al. (2020) reported peak fawning to occur May 28th, with the earliest and latest births recorded on May 9th and June 23rd, respectively. Tomberlin et al. (2007) captured fawns within 150 km of Howard County, MD, between May 24 and June 8 and used those dates to calculate likely breeding dates ranging from November 5 - November 25. Four of the county parks did have sporadic deer management, including sharpshooting or managed hunting. However, all culling efforts occurred ≤ 6 days per park in a given year, and every park was not culled every year. When conducted, sharpshooting was implemented at night over bait piles by licensed marksmen and was typically scheduled during the state hunting season. However, as of 2018, all culling was further restricted to February - March. Managed hunts were restricted to shotgun and archery hunting by registered public participants from October - January. Middle Patuxent Environmental Area, Blandair Regional Park, and Wincopin Trails System all had annual managed hunts since 2014, and Savage Park, Rockburn Branch Park, and Blandair Regional Park had sharpshooting since 2007. Population management was not conducted in Cedar Lane Park (Online Resource 1, Table S1). Hunters were asked to refrain from killing collared deer during active data collection. Once deer GPS collars were ready to be retrieved, hunters were encouraged to harvest collared deer. Additionally, many Howard County parks have also done some level of tick management via deer. During our study period, 4-poster tick-control feeders were available to deer at Cedar Lane Park, Blandair Regional Park, and Rockburn Branch Park. These integrated pest management devices were implemented at densities of 1 feeder per 15–19 ha (Pound et al. 2000). Feeders were removed from the field typically from January-February each year to avoid interference with hunting operations. These 4-poster feeders inherently feed wildlife to enable tickicide application. There was also anecdotal evidence of wildlife feeding conducted by local residents at every park during this project. While inconsistent, these county-level deer culling efforts and tick mitigation management schemes were typical for this region.
Trapping methods
We captured deer between January and April in 2017 and 2018 using drop nets (15.2 m x 15.2 m) and box traps (0.9 m width x 1.22 m height x 1.83 m length; Wildlife Capture Services, Flagstaff, AZ) baited with whole kernel corn and apples (Peterson et al. 2003). We used four box traps total, rotating them among parks, and hid them from human view to reduce interference. We set box traps in the evening and checked them once a day at dawn. We selected drop net placement in each park to reduce interference with human recreational activity while maintaining ease of vehicle access (Roden-Reynolds et al. 2020). When an animal was identified under a drop net, the field crew activated or dropped the net, and physically restrained the animals. After capture, we anaesthetized animals by hand syringe in the gluteal muscle mass using BAM™ (Wildlife Pharmaceuticals, Windsor, CO; McDermott et al. 2020). The fixed-dose BAM™ formulation contained 27.3 mg of Butorphanol, 9.1 mg of Azaperone, and 10.9 mg of Medetomidine per 1 ml of solution. We administered BAM™ based on visually estimated weight according to label directions. After injection, we applied face blinds, and moved deer to a ground tarp for processing. During the processing period, we sexed each individual and estimated age by examining tooth wear and replacement (Severinghaus 1949). We deployed Lotek GlobalStar L collars (satellite GPS collars with VHF beacon, 850 g) on individuals deemed ≥ 1 year of age with sufficient neck circumference of ≥ 30.0 cm. Collars for males had expandable mechanisms allowing collar circumference to increase from general growth and during breeding periods. Often collars were retrofitted with foam and tape to reduce the collar shifting on the neck and subsequent irritation (Collins et al. 2014). After a minimum 20-min processing period, we reversed BAM™ with intramuscular administration of Atipamezole (25 mg/ml) and Naltrexone (50 mg/ml) (Wildlife Pharmaceuticals, Windsor, CO) in amounts based on the initial injection volume of BAM™. Manufacturer recommends a reversal of 0.5 ml (25 mg) of Naltrexone for all set doses of BAM™, and at least 1.0 ml (25 mg) of Atipamezole for every 0.5 ml of BAM™ administered. We immediately released deer after recovery and monitored them until they exited the area. We monitored newly collared deer via VHF daily for the first three days after deployment and then biweekly to assess collar functioning and deer activity.
GPS collars remained on for a pre-programmed duration (~ 116 or 62 weeks, depending on deployment date) and recorded a GPS location and timestamp onboard every hour. We formatted GPS timestamps to Eastern Standard Time. GPS collars also attempted to remotely upload a subset of locations to a cloud service every third hour. Collars were equipped with dual-axis accelerometers that recorded motion in the x- and y-axes detecting forward and backward motion and sideways or rotary motion. Activity was recorded simultaneously on each axis (Activity X and Activity Y) as the difference in acceleration (rate of change in velocity) between two consecutive measurements and recorded across a relative scale of 0 and 255. While activity was recorded every five minutes, we analyzed only the subset of activity values that occurred simultaneously with the hourly GPS locations. This activity data was not a direct measurement of acceleration or movement but an index of change in motion, where high activity values indicated more change in motion and low activity resulted in less change in motion between simultaneous recordings. Prior to initial collaring, we deployed one GPS collar at six known locations of varying habitat cover for a minimum of 3 days to estimate average locational error of our collars.
Broad-scale analysis
To quantify variation in space utilization at broad spatial and temporal scales we calculated annual and seasonal home range estimates at two scales. We created annual home ranges for each individual that had at least 10 months of data available from the deployment date (Kilpatrick et al. 2011). Separate home ranges for summer (June 21st -September 22nd ) and winter (December 21st -March 20th ) were created if the dataset from each deer fully overlapped those dates. Both annual and seasonal ranges were calculated at the 95% home range and 50% core range levels. All range contours were calculated with autocorrelated kernel density estimators (AKDE) using ctmmweb (Fleming et al. 2015; Calabrese et al. 2016; Dong et al. 2018). The AKDE method accounted for autocorrelation of our large, hourly-resolution location datasets, generating a less biased estimation of home ranges than traditional kernel methods (Fleming et al. 2014, 2015). To account for telemetry error and calibrate datasets, we manually input User Equivalent Range Error values within ctmmweb using the average locational error of 10 m established from our field-tested collar units. When individual collars were not recovered, we utilized the remotely uploaded datasets. However, the remote datasets often contained missing data leading to variable gaps in sampling frequency, therefore, we analyzed those datasets by enabling optimal weighting. Optimal weighting applied weights to locations based on temporal sampling bias to correct for discrete time periods with oversampling (Fleming et al. 2018). White-tailed deer are typically resident species but did exhibit some individual shifting of their home ranges in this region, which could have resulted in poor home range estimation (Rhoads et al. 2010; Calabrese et al. 2016). As such, the autocorrelation structure of each annual or seasonal home range dataset was visualized using variograms, and we excluded any home range whose variogram did not reach an asymptote (Fleming et al. 2014; Calabrese et al. 2016). Then, if an individual deer still had multiple annual home ranges or two of the same seasonal home ranges, the first complete annual or seasonal home range was retained for further analysis.
To characterize deer home range placement on the landscape, we analyzed land ownership and the amount of residential land within the 95% and 50% home range contours using ArcGIS and Howard County GIS Land Use layer (Howard County 2015; Online Resource 1, Table S2). Next, we tabulated the number of residential properties within the 50% core range contours in ArcGIS using a property boundaries data layers (Howard County 2015). When categorizing apartment buildings, those properties were treated as one residential unit because they shared a single property, with a continuous property boundary. To enable comparisons between parks, the land use composition and residential building density were calculated within a circle around each drop-net site. We buffered each drop-net site using a radius equal to the average cumulative distance moved by deer each day (2,145 m). Finding the data to be non-normal, we used Wilcoxon rank sum tests to compare home range size, housing density within core ranges, land use within ranges, with data grouped and averaged by season, sex, or both depending on the analysis.
Fine-scale analysis
To quantify how deer moved at a fine spatial and temporal scale, only full datasets with GPS locations recorded at 1 h intervals were included to decrease any fix rate bias (Pépin et al. 2004; Rowcliffe et al. 2012; Massé and Côté 2013). Then, we removed the initial 14 days of each deer’s GPS dataset to reduce any potential bias caused from capture and collaring (Dechen Quinn et al. 2012). We measured the Euclidean distance and time between successive points to determine the minimum hourly recorded deer speed (meters/hour). To assess activity, both Activity X and Activity Y axes were summed to a single activity score as they were highly correlated (Edmunds et al. 2018). The activity data was heavily zero-inflated and was transformed into a Bernoulli variable, where an activity score greater than one was coded as 1 and score less than one coded as 0. Moreover, though past studies have used this activity data to classify different behaviors, studies seeking to judge the accuracy of such metrics find that there was significant overlap between different behaviors and their associated activity states (Coulombe et al. 2006). As such, simplifying this variable to active and inactive constituted a more conservative approach and provided greater confidence in any results. Lastly, we measured the Euclidean distance from GPS locations of deer to the nearest residential building using the land use and building data layers (Howard County 2015).
We analyzed sex-specific ultradian and infradian patterns in speed, activity, and distance to residential buildings with general additive models (GAM) using package mgcv and the function bam(). All models contained smooth tensor-product interactions between hour of day, day of year, and sex, all lower-order interactions, and an independent identically distributed random effect of individual deer. All smooth terms used cyclic penalized cubic regression splines and smooth parameter selection was done using fast restricted maximum likelihood (fREML). Within this framework, model selection is performed automatically for the smoothing parameter to prevent overfitting the data and prevent producing a model that is too “wiggly” (Wood 2004). Speed was modeled with a gamma distribution and a log link, and the small number (7 out of 209,703) of observations that were exactly 0 were excluded from analysis. Activity was modeled with a binomial distribution and logit link. We also attempted to model the raw activity scores with a zero-inflated Poisson model, which found similar results but failed to meet model assumptions so was not included. When modeling distance to the nearest residential building, we encountered strong residual temporal autocorrelation. Due to distribution constraints with bam() when including temporal autocorrelation, distance to building was normalized with a square root-transformation and an autoregressive (AR1) autocorrelation structure was included. Significance of all three models was assessed with an analysis of variance (ANOVA) and the adjusted R2 value was calculated to assess the proportion of variance explained. All analyses were conducted in R Version 4.0.2 (R Core Team 2020).
Results
GPS Data
We collected locational data from 51 deer (33 females, 18 males), with an average estimated age of 2.7 ± 0.9 years (Range: 1–5). This included 13 deer collared at Cedar Lane Park, 10 at Blandair Regional Park, 9 at Middle Patuxent Environmental Area, 9 at Rockburn Branch Park, and 10 at Wincopin Trails System. During the study, roadkill was the greatest source of documented mortality (n = 8), followed by hunter harvest (n = 5) and unknown mortality sources (n = 2). We recovered 27 hourly store-on-board datasets. Malfunctions and drained batteries prevented recovery of 24 collars, limiting those collars to the locations that had remotely transmitted to the online database. As such, only the 27 deer (15 female/12 male) with successful hourly fix rates were used for fine scale speed, activity, distance to residential buildings analyses. Prior to home range analyses of the locational dataset, we excluded 5 seasonal and 7 annual home range estimates, from 11 unique individuals (7 males, 4 females), due to non-asymptotic variograms. After exclusion of any duplicate ranges for an individual, 14 individual deer had annual home ranges, 32 deer had summer ranges, and 14 had fall seasonal ranges (Table 1).
Broad-scale home ranges
Annual and winter home ranges did not differ among parks, but in summer, deer at Cedar Lane had larger home ranges than at Rockburn and Blandair (95% home range: X2 = 13.27, df = 4, p-value = 0.01; 50% core ranges: X2 = 13.68, df = 4, p-value = 0.008). Due to lack of data from some parks, park datasets were combined and analyzed as one unit representing suburban parks in the county. Average home range size was variable across sexes and seasons (Table 1). Summer home and core range sizes were significantly smaller than winter ranges for both sexes, while the difference in annual 95% range sizes approached significance between sexes (Table 2).
Parks and residential land were the dominant land use classes within home ranges across all years and seasons (Fig. 2). Other minor land use classes included institutional land (e.g. school grounds, cemeteries) and undeveloped land. A greater proportion of park land was found within core ranges whereas more residential land was within the broader home ranges for both seasons. More residential land was used during winter months; however, this pattern was not statistically significant (Table 2). Specific study area land use compositions are available in Online Resource 1, Table S3.
Proportion of land use and standard deviation error bars within white-tailed deer home range 95% and core range 50% contours for combined sexes across seasons in Howard County, Maryland 2017–2019. Land use classes are reclassified from Howard County (2015) and defined in Online Resource 1, Table S2
When comparing the average housing density (residential buildings/ha) in annual core ranges for females (3.36 ± 2.62 homes) and males (2.16 ± 1.88 homes, Table 3), no significant difference was found (W = 305, p-value = 0.10). We found higher average housing densities within winter core ranges than summer core ranges but densities were not significantly different for either sex, although the difference for males approached significance (Table 2). Specific park study area housing density is available in Online Resource 1, Table S3.
Fine-scale movements
The three-way interaction between hour of day, day of year, and sex was a significant predictor of speed, activity, and distance to residential buildings and all lower-order effects were retained for all fine-scale models (Table 4), though, as expected, the proportion of variance explained was generally low (R2 = 0.08, 0.08, and 0.30 respectively). Both females and males greatly increased speed during periods immediately following sunrise and sunset, but the magnitude of speed differed among parts of the year with greatest speeds occurring in non-summer months (Fig. 3a). Speed increased in winter compared to summer for both sexes, but females did exhibit greater speeds in the summer, and males showed much greater speeds during late autumn, especially during nighttime hours (Online Resource 1, Fig. S1).
Model predictions for speed, activity, and distance to residential buildings by hour of day and day of year for female and male white-tailed deer in Howard County, Maryland 2017–2019, ignoring the random effect of individual. Curved, dashed lines denote sunrise and sunset. (a) Depicts speed (meters/hour), (b) depicts proportion of active deer, (c) depicts distance to residential buildings. The smoothness parameter was selected automatically during model fitting, and the three-way interaction between time, date, and sex was significant for all models
Activity peaked within the hour immediately following sunrise and sunset but rebounded throughout the day particularly during the summer, with diurnal activity decreasing during winter (Table 4; Fig. 3b). Distinct resting periods of decreased activity were identifiable throughout the year shortly after morning peaks in speed and activity. Differences in female and male activity were strongest from June to November when female diurnal activity was greater and males exhibited more bouts of rest (Online Resource 1, Fig. S2).
Both males and females moved towards residential buildings during nighttime hours and further away during the day (Table 4). Regardless of the time of year, deer began to steadily move towards residential areas around 17:00, with proximity to buildings peaking around 4:00, and having fully returned to the maximum distance from buildings by 8:00. In general terms, deer were twice as close to residential properties at night. Although the exact distances were highly variable throughout the year and by individual deer, for context, the average raw data shifted from around 100-120 m from residential properties during days in April and May and to around 40-60 m during nights in December and January (Fig. 3c). Additionally, both sexes were further from residential buildings during spring (Fig. 3c), with males avoiding residential areas from November to December (Online Resource 1, Fig. S3).
Discussion
In the face of increasing tick-borne disease issues as well as general overpopulation of deer in suburban and urban areas, the results of our study help facilitate more effective guidelines for limiting deer population numbers and movement, and by extension influencing tick distribution in residential landscapes (Kilpatrick et al. 2014). Generally, deer avoided residential areas during the day, with core ranges primarily encompassing park lands. These nightly movements became more obvious during the winter months, with expanded home ranges that included even more residential properties. Below, we discuss several sex-specific trends related to life-history patterns that are relevant to our management recommendations. Finally, by integrating both broad and fine-scale spatial patterns of deer with the life cycle of the blacklegged tick, the primary vector of Lyme and other tick-borne diseases in the eastern United States, we provide specific management recommendations for residential areas concerned about Lyme disease or other tick-borne pathogens.
Deer spatial Ecology
Our home and core range sizes, though smaller than studies done in rural or exurban areas, were still larger than many past studies involving urban and suburban deer (Kilpatrick and Spohr 2000a, b; Etter et al. 2002; Grund et al. 2002; Kilpatrick et al. 2011). This is likely due to past use of fixed or adaptive kernel methods (Storm et al. 2007; Walter et al. 2009). We used autocorrelated kernel density estimators that are better able to assess space use with autocorrelated data, typical of high-resolution GPS data. At the same time, AKDE can be sensitive to any significant shifting of space use, often a characteristic of dispersing animals or bimodal home ranges, which could result in an overestimate of space use. However, bimodal home ranges can naturally arise from deer exploiting disjointed patches in highly fragmented suburban and urban landscapes, resulting in multiple home range centers. In fact, such home ranges have been reported in Maryland, with distances as great as 6 km between home range centers (Eyler 2001; Rhoads et al. 2010). While we chose to be conservative in what was included, we suggest researchers carefully consider the implications of variogram structures and data exclusion when using AKDE in highly fragmented suburban and urban habitats.
Similar to past work, we saw high variability in home range size across individuals (Kilpatrick et al. 2011), likely arising from factors such as age, sex, social status, or population density. Each of those factors could influence individual space use on the landscape during relevant seasons such as mating or parturition. In our study, home ranges of white-tailed deer predominantly contained park land; but residential land comprised a substantial portion at each home range level (Fig. 2). Regardless of sex, we found a much greater number of individual residential properties within deer core ranges compared to past research (Kilpatrick and Spohr 2000a, b; Storm et al. 2007; Kilpatrick et al. 2011). While average male core ranges contained more residential properties than females (Table 3), that was likely a byproduct of males having larger home ranges. In fact, female deer core ranges contained greater average housing densities, and females were consistently closer to residential buildings. Thus, this study illustrated that one individual deer had the potential to interact with hundreds of residential properties, emphasizing their potential for human conflict and transporting ticks and other parasites into yards.
Multiple analyses pointed to an increase in the use of residential areas and related changes in home range size during the winter months. Although not statistically significant, as home ranges increased size during winter months, we saw a corresponding increase in proportion of residential land and decrease in park land within ranges. Consistent with this broadly observed pattern, in our fine-scale hourly movement data, we documented that nightly movements of deer into residential areas increased during winter. In contrast to winter, April through June marked months when both sexes were furthest from residential buildings in our fine-scale movement data as well as documented reduction in summer home range sizes. Both the increased distance from homes and reduction in home range size were likely related to the increased forage available and females encumbered by fawns, enabling or requiring deer to travel less (Long et al. 2008; Walter et al. 2011; Massé and Côté 2013).
Several past studies have highlighted the increased use of residential areas during winter months (Kilpatrick and Spohr 2000a; Grund et al. 2002; Storm et al. 2007), and suggested deer may be exploiting ornamental plants, bird feeders, and supplemental feed placed by residents (Williams and Ward 2006). We acknowledge that supplemental feed was available in our study in two forms and may have influences general deer movements. First, a subset of collared deer had home ranges within park areas where 4-poster integrated tick control feeders were deployed during spring and summer, and secondly, baiting by residents using corn and mineral licks for purposes of wildlife viewing was documented in several locations on our study areas, some on private land and some on public. While we acknowledge that both practices may have had some impact on deer movements or home range placement, our analyses, while more precise, followed similar patterns of past studies and the expansion of home ranges in winter did not occur in autumn when feeders were not deployed (Kilpatrick and Spohr 2000a, b; Grund et al. 2002; Storm et al. 2007; Kilpatrick et al. 2011; Potapov et al. 2014).
Interestingly, deer distances to residential buildings did not closely track with changes in the timing of sunrise and sunset, perhaps because deer were responding to reduced human activity levels. Human schedules are likely determined by school or work, less directly linked to photoperiod, although still impacted by changing between eastern standard time in winter and daylight savings time in summer. For example, during mid-summer, deer did not begin to leave residential areas until well after sunrise. Even though Maryland clocks had been shifted forward an hour from mid-March through October, that was not enough to fully offset the approximate 2 h earlier that the sun was rising in mid-summer, allowing deer to linger as the sun rose and before human activity increased.
Unlike proximity to residential buildings and similar to Rhoads et al. (2010), we documented that speed and activity consistently increased directly after sunrise and sunset throughout the year and the dawn peak was more evident during non-winter months (Fig. 3a). Clear resting and likely bedding periods (low speed, low activity) directly followed these morning peaks, perhaps due to ruminating behavior after foraging events (Massé and Côté 2013). However, following these resting periods, we documented another increase in activity levels, potentially related to resumed foraging. Additionally, in contrast to summer fine-scale movements, we recorded a decrease in daytime winter activity but an increase in overall winter movements. These trends can likely be attributed to changing distribution of forage and cover, but contrary to what Massé and Côté (2013) documented, we saw an inverse relationship between summer and winter movement and activity. Specifically, in winter, deer likely moved farther from place to place looking for forage, but less small daily movements and more bedding occurred due to reduced resources and conservation of energy behaviors. Meanwhile, in summer, less large movements between foraging patches were needed but more small daily movements occurred (Massé and Côté 2013).
It should be noted that our fine-scale models of speed and activity explained only a small amount of the overall variance. This is unsurprising, given that these behaviors are likely driven by very specific events (e.g., interactions with homeowners, park users, or pets). Nevertheless, we documented changes in speed and activity across time, with changes that were both statistically significant and of high magnitude. Therefore, though our models were unable to accurately predict individual deer movements at a given hour on a given date, they identify real trends with large differences in average overall deer movement (Matloff 2017). As such, while these analyses illustrated the need for more work on specific behavioral responses to specific events, we were able to document clear trends in these patterns which help to illustrate how deer utilize and move through suburban and urban landscapes. Moreover, our model explained a much higher proportion of the variance in distance to residential buildings, underscoring the consistency of these seasonal and nightly movements. Lastly, it should also be noted that though these models are based on a large number of hourly data points, these come from only 27 individual deer. Our repeated measures design allows for great statistical power when examining the temporal patterns of individual deer. However, some care is warranted in extrapolating baseline estimates outside of this study because of the small number of collared deer.
Population Management
All previously discussed results have implications for the many natural resource agencies that use visual or camera surveys to collect data on deer densities in suburban areas, which is crucial for effective deer management. Over the last few decades, deer management has largely been focused on public properties, where management of populations is more feasible (Peterson et al. 2003). Given the number if residential properties deer encounter as well as the significant portion of general deer space use on private lands (e.g. homeowner properties, school grounds, religious facilities), accounting for and better including privately-owned lands could greatly increase suburban deer management effectiveness. Our research shows deer space use changes depending on time of day and time of year. As such, any survey should sample both residential and park areas simultaneously, when feasible. Many visual demographic surveys are often conducted at night due to increased visibility of deer. Our data shows nighttime estimates in residential areas may overestimate abundance, while nighttime estimates from park land may underestimate them. Any sampling design for deer population estimates in highly suburban or urban areas with larger patches of park forest land should account for daily and seasonal cycles in space use.
Aside from accurate density estimates, agencies need access to a majority of individuals from a population when conducting population control. (Kilpatrick et al. 2002; Telford 2017). Hunting has been perceived as best during twilight periods because generally deer are moving more during these periods; however, any increase in diurnal speed or activity can increase chance encounters with hunters. Although this study supports those peaks in speed immediately following sunrise and sunset, we documented deer do not generally rest throughout the main parts of the day. We saw a strong midday peak in activity, especially during summer months, with midday speeds also increasing for males during mating periods and late winter. Additionally, the ‘October lull’ has anecdotally been described by hunters as a period of low movement rates and activity in white-tailed deer during the month of October, but previous research has generally not supported this phenomenon (Tomberlin 2007; Simoneaux et al. 2016). We did document evidence for a lull in daytime speed and activity for males during October, but when compared to previous months, the daytime lull coincided with an overall increase in speed and activity just after sunrise and sunset and through the night. So, managers may wish to avoid planning any daytime hunts earlier than mid-October in this region and continue to focus managed hunting efforts on park property, typically one half hour before sunrise to one half hour after sunset.
Unfortunately, although regulations vary by county, safe locations for such hunting or sharpshooting in suburban areas are generally highly limited. For example, the required distance from occupied residential property in much of the state of Maryland exceeds 91 m for archery hunting, yet 66% of our deer locations were closer than 91 m to residential buildings. Frequently reassessing hunting safety zones when feasible and encouraging hunting methods that utilize archery equipment could increase efficiency. Lastly, sharpshooting operations often occur at night in suburban or urban park properties as a more discrete and efficient method to reduce deer populations. However, our study shows that deer often move out of park areas and into residential yards at night. Furthermore, this movement of deer into residential yards is often intensified during typical hunting months, even in areas that are not routinely harvested. With appropriate sharpshooting tactics and permissions, culling closer to residential areas in fall and winter, including transitional areas between designated parks and residential properties such as pipelines, riparian buffers, county open space or undeveloped lots, would likely increase the effectiveness of population reduction programs, especially when managers choose to target female deer.
Deer movements and Tick Life cycles
Tick-borne diseases represent major public and wildlife health concerns across the world, and ticks are a principal vector of the causative agent of many zoonotic diseases. This is especially true for the eastern U.S., where suburban and urban habitats support high densities of deer that are important to blacklegged and lone star tick reproduction and dispersal (Kollars et al. 2000; Huang et al. 2019; Milholland et al. 2021a, b). Given that chances of people becoming exposed to tick bites and tick-borne disease is highest in their own yards, we must consider the direct and indirect influences on tick populations around people’s homes (Stafford et al. 2017). For example, when focusing on blacklegged ticks and Lyme disease, there are two primary tick life cycle stages to consider: nymphs and adults. Nymph ticks and adult ticks can both bite and transmit disease to humans, and adult ticks can lay eggs in residential areas, increasing or establishing a tick population. For Lyme disease, the dominant tick-borne disease in Maryland, the nymph stage is considered the primary vector of the spirochetal infection (Feldman et al. 2015: Millholland et al. 2021a). These nymphs feed during the spring and summer months, are very small, and often not easily detected. In a recent study in Maine, there was evidence of a relationship between higher deer densities supporting more blacklegged tick nymphs (Elias et al. 2021). Furthermore, despite warmer winters, nymph numbers only increased after a threshold of deer was exceeded (Elias et al. 2021). That study suggested that deer population reductions could be one key factor in combating the expected blacklegged tick increases associated with climate change (Elias et al. 2021).
Adult blacklegged ticks can also transmit Lyme disease, but they are larger and easier to see and remove before they transmit the bacteria. In Maryland, the adult blacklegged tick becomes very active in October and November (Orr et al. 2013). Fall and winter months are high risk times for deer transporting adult ticks into residential areas, with female deer posing the greatest risk of increasing adult ticks near homes. Adult female ticks may then fall off deer near residential properties and mate and lay eggs in early spring. This increase in use by deer around residential areas during winter months combined with prolonged tick activity and lessened tick mortality due to climate change may increase or intensify successful hatching of tick larvae in or around people’s properties (Ogden and Lindsay 2016; Dumic and Severnini 2018).
While deer use of residential areas during summer is less intense than winter, the majority of deer in our study still placed approximately 35% of their home ranges in residential spaces. Increased tick activity during summer coinciding with human outdoor activity likely leads to increased risk of encountering ticks, both nymph and adult. If applicable, residential property owners should utilize the commonly recommended tick management strategies (Stafford 2007; Stafford et al. 2017), such as proper landscape management, vector host management, and biological or chemical control for their property. However, we suggest that all landscape management and management of areas of shade and moisture should be reassessed up to 40 m away from the residence, as applicable. Whereas most recommendations offer up such distances such as 1-meter perimeter bed of wood chips to delineate the forest edge and create a barrier to ticks (Stafford 2007), deer carrying ticks step right over these measures in many yards. So, in areas with a high prevalence of Lyme disease, we recommend taking more significant precautions to reduce the likelihood of deer entering areas of private yards that will have high overlap of human activity such as gardening, yardwork, and recreational activity. As suggested by Stafford (2007) and others, we too recommend reducing deer access to any anthropogenic food sources such as bird feed and garden residues as well as securing compostable items such as vegetable rinds, especially during winter months. Furthermore, baiting, although discouraged by natural resource agencies, is not strictly illegal in Maryland for most of the year. Legislation to outlaw wildlife feeding in metropolitan areas not associated with hunting or other management activities could aid in the reduction of deer presence on private properties. In very high Lyme prevalence areas, we recommend strong consideration of fencing. Fencing is a proven option to exclude deer but may be cost prohibitive depending on design, and high fencing may be restricted or unappealing in many suburban and urban developments.
Conclusions
An overabundance of white-tailed deer in suburban and urban areas is one facet of the complex ecology of Lyme disease and likely other tick-borne zoonotic diseases in these unique environments. Deer contribute to the maintenance of tick populations carrying zoonotic diseases in the environment and that potential will likely grow without human intervention. Here, we have deepened the understanding of the spatial ecology of deer and their behaviors in suburban areas, including movement differences between sexes, time of day, and across seasons. We have documented some of the highest number of residential properties within deer core ranges and illustrated how that residential space use expands in winter. We provide information on home range, speed, activity, and distance to residential buildings that can be used to inform ongoing management and future research, especially as it pertains to risks associated with yard spaces used by deer at specific times. Insights into how these findings might be utilized to improve over-abundant deer population management for the health of the ecosystem as well as to help reduce tick populations in residential yards have been provided. This work highlights the importance of continuing research on urban and suburban deer ecology, across scales and through the lens of management of zoonotic diseases.
Data Availability
Not Applicable.
Code Availability
Not Applicable.
References
Allan BF, Goessling LS, Storch GA, Thach RE (2010)Blood Meal Analysis to Identify
Reservoir Hosts for Amblyomma americanum Ticks.Emerg Infect Dis16:433–440. https://doi.org/10.3201/eid1603.090911
Calabrese JM, Fleming CH, Gurarie E (2016) ctmm: an R package for analyzing animal relocation data as a continuous-time stochastic process. Methods Ecol Evol 7:1124–1132. https://doi.org/10.1111/2041-210X.12559
Collins GH, Petersen SL, Carr CA, Pielstick L (2014) Testing VHF/GPS Collar Design and Safety in the Study of Free-Roaming Horses. PLoS ONE 9. https://doi.org/10.1371/journal.pone.0103189
Coulombe ML, Massé A, Côté SD (2006) Quantification and accuracy of activity data measured with VHF and GPS telemetry. Wildl Soc Bull 34:81–92. https://doi.org/10.2193/0091-7648(2006)34[81:QAAOAD]2.0.CO.1
Crispell G, Commins SP, Archer-Hartman SA et al (2019) Discovery of Alpha-Gal-containing antigens in North American tick species believed to induce red meat allergy. Front Immunol 10:1056. https://doi.org/www.frontiersin.org/article/10.3389/fimmu.2019.01056
Dechen Quinn AC, Williams DM, Porter WF (2013) Landscape structure influences space use by white-tailed deer. J Mammal 94:398–407. https://doi.org/10.1644/11-MAMM-A-221.1
Dechen Quinn AC, Williams DM, Porter WF (2012) Postcapture movement rates can inform data-censoring protocols for GPS-collared animals. J Mammal 93:456–463. https://doi.org/10.1644/10-MAMM-A-422.1
DeNicola AJ, Williams SC (2008) Sharpshooting suburban white-tailed deer reduces deer–vehicle collisions. Hum-Wildl Confl 2:28–33
Dion JR, Haus JM, Rogerson JE, Bowman JL (2020) White-tailed deer neonate survival in the absence of predators. Ecosphere 11:e03122. https://doi.org/10.1002/ecs2.3122
Dong X, Fleming CH, Noonan MJ, Calabrese JM(2018) ctmmweb: A Shiny web app for the ctmm movement analysis package. https://github.com/ctmm-initiative/ctmmweb. Accessed 07 March 2021
Dumic I, Severnini E (2018) “Ticking Bomb”: The Impact of Climate Change on the Incidence of Lyme Disease. Can J Infect Dis Med Microbiol 2018:1–10. https://doi.org/10.1155/2018/5719081
Edmunds DR, Albeke SE, Grogan RG et al (2018) Chronic wasting disease influences activity and behavior in white-tailed deer: Effects of CWD on Behavior of Deer. J Wildl Manag 82:138–154. https://doi.org/10.1002/jwmg.21341
Elias SP, Gardner AM, Maasch KA et al (2021) A Generalized Additive Model Correlating Blacklegged Ticks with White-Tailed Deer Density, Temperature, and Humidity in Maine, USA, 1990–2013. J Med Entomol 58:125–138. https://doi.org/10.1093/jme/tjaa180
Etter DR, Hollis KM, Van Deelen TR et al (2002) Survival and Movements of White-Tailed Deer in Suburban Chicago, Illinois. J Wildl Manag 66:500–510. https://doi.org/10.2307/3803183
Eyler TB (2001) Habitat use and movements of sympatric sika deer (Cervus nippon) and white-tailed deer (Odocoileus virginianus) in Dorchester County, Maryland. University of Maryland, Eastern Shore
Feldman KA, Connally NP, Hojgaard A et al(2015) Abundance and infection rates of Ixodes scapularis nymphs collected from residential properties in Lyme disease-endemic areas of Connecticut, Maryland, and New York. J Vector Ecol 40:198–201
Fleming CH, Calabrese JM, Mueller T et al (2014) From Fine-Scale Foraging to Home Ranges: A Semivariance Approach to Identifying Movement Modes across Spatiotemporal Scales. Am Nat 183:E154–E167. https://doi.org/10.1086/675504
Fleming CH, Fagan WF, Mueller T et al (2015) Rigorous home range estimation with movement data: a new autocorrelated kernel density estimator. Ecology 96:1182–1188. https://doi.org/10.1890/14-2010.1
Fleming CH, Sheldon D, Fagan WF et al (2018) Correcting for missing and irregular data in home-range estimation. Ecol Appl 28:1003–1010. https://doi.org/10.1002/eap.1704
Frank DH, Fish D, Moy FH (1998) Landscape features associated with lyme disease risk in a suburban residential environment. Landsc Ecol 13:27–36. https://doi.org/10.1023/A:1007965600166
Grund MD, McAninch JB, Wiggers EP (2002) Seasonal Movements and Habitat Use of Female White-Tailed Deer Associated with an Urban Park. J Wildl Manag 66:123–130. https://doi.org/10.2307/3802878
Howard County Department of Planning and Zoning, Research Division (2020) Howard County Demographic Overview. https://www.howardcountymd.gov/planning-zoning/demographic-socioeconomic-data. Accessed 07 March 2021
Howard County GIS(2015) Data Download and Viewer. Howard County Government. Howard County, Maryland. Department of Technology and Communications, The Geographic Information Systems (GIS) Division. https://data.howardcountymd.gov/. Accessed 07 March 2021
Huang C-I, Kay SC, Davis S et al (2019) High burdens of Ixodes scapularis larval ticks on white-tailed deer may limit Lyme disease risk in a low biodiversity setting. Ticks Tick-Borne Dis 10:258–268. https://doi.org/10.1016/j.ttbdis.2018.10.013
Hussain A, Armstrong JB, Brown DB, Hogland J (2007) Land-use pattern, urbanization, and deer–vehicle collisions in Alabama. Hum-Wildl Confl 1:89–96
Kilpatrick HJ, Labonte AM, Barclay JS (2011) Effects of landscape and land-ownership patterns on deer movements in a suburban community. Wildl Soc Bull 35:227–234. https://doi.org/10.1002/wsb.48
Kilpatrick HJ, LaBonte AM, Seymour JT (2002) A Shotgun-Archery Deer Hunt in a Residential Community: Evaluation of Hunt Strategies and Effectiveness. Wildl Soc Bull 1973–2006 30:478–486
Kilpatrick HJ, Labonte AM, Stafford KC III (2014) The Relationship Between Deer Density, Tick Abundance, and Human Cases of Lyme Disease in a Residential Community. J Med Entomol 51:777–784. https://doi.org/10.1603/ME13232
Kilpatrick HJ, Spohr SM (2000a) Movements of Female White-Tailed Deer in a Suburban Landscape: A Management Perspective. Wildl Soc Bull 4:28:1038–1045
Kilpatrick HJ, Spohr SM (2000b) Spatial and Temporal Use of a Suburban Landscape by Female White-Tailed Deer. Wildl Soc Bull 4:28:1023–1029
Kollars TM Jr, Oliver JH Jr, Durden LA, Kollars PG (2000) Host Associations and Seasonal Activity of Amblyomma americanum (Acari: Ixodidae) in Missouri. J Parasitol 86:1156–1159. https://doi.org/10.1645/0022-3395(2000)086[1156:HAASAO]2.0.CO;2
Kraft J(2008) Soil Survey of Howard County, Maryland. Natural Resources Conservation Service. https://www.nrcs.usda.gov/Internet/FSE_MANUSCRIPTS/maryland/MD027/0/MDHoward5_08.pdf. Accessed 07 March 2021
LoGiudice K, Duerr STK, Newhouse MJ, Schimidt KA, Killilea ME, Ostfeld RS (2008) Impact of host community composition on Lyme disease risk. Ecology 89(10):2841–2849
Long ES, Diefenbach DR, Rosenberry CS, Wallingford BD (2008) Multiple proximate and ultimate causes of natal dispersal in white-tailed deer. Behav Ecol 19:1235–1242. https://doi.org/10.1093/beheco/arn082
Massé A, Côté SD (2013) Spatiotemporal variations in resources affect activity and movement patterns of white-tailed deer (Odocoileus virginianus) at high density. Can J Zool 91:252–263. https://doi.org/10.1139/cjz-2012-0297
Matloff NS (2017) Statistical regression and classification: from linear models to machine learning. CRC Press, Boca Raton
McDermott JR, Leuenberger W, Haymes CA et al (2020) Safe Use of Butorphanol–Azaperone–Medetomidine to Immobilize Free-Ranging White-tailed Deer. Wildl Soc Bull 44:281–291. https://doi.org/10.1002/wsb.1096
Milholland MT, Eisen L, Nadolny RM et al (2021a) Surveillance of Ticks and Tick-Borne Pathogens in Suburban Natural Habitats of Central Maryland. J Med Entomol. https://doi.org/10.1093/jme/tjaa291
Milholland MT, Xu G, Rich SM et al (2021b) Pathogen Coinfections Harbored by Adult Ixodes scapularis from White-Tailed Deer Compared with Questing Adults Across Sites in Maryland, USA. Vector-Borne Zoonotic Dis 21:86–91. https://doi.org/10.1089/vbz.2020.2644
Ogden NH, Lindsay LR (2016) Effects of Climate and Climate Change on Vectors and Vector-Borne Diseases: Ticks Are Different. Trends Parasitol 32:646–656. https://doi.org/10.1016/j.pt.2016.04.015
Orr JM, Smith JD, Zawada SG, Arias JR (2013) Diel and seasonal activity and trapping of ticks (Acari: Ixodidae) in Northern Virginia, U.S.A. Syst Appl Acarol 18:105. https://doi.org/10.11158/saa.18.2.1
Pépin D, Adrados C, Mann C, Janeau G (2004) Assessing Real Daily Distance Traveled by Ungulates Using Differential GPS Locations. J Mammal 85:774–780. https://doi.org/10.1644/BER-022
Peterson MN, Lopez RR, Frank PA et al (2003) Evaluating Capture Methods for Urban White-Tailed Deer. Wildl Soc Bull 1973–2006 31:1176–1187
Porter WF, Underwood HB, Woodard JL (2004) Movement Behavior, Dispersal, and the Potential for Localized Management of Deer in a Suburban Environment. J Wildl Manag 68:247–256. https://doi.org/10.2193/0022-541X(2004)068[0247:MBDATP]2.0.CO;2
Potapov E, Bedford A, Bryntesson F et al (2014) White-Tailed Deer (Odocoileus virginianus) Suburban Habitat Use along Disturbance Gradients. Am Midl Nat 171:128–138. https://doi.org/10.1674/0003-0031-171.1.128
Pound JM, Miller JA, George JE, Lemeilleur CA (2000) The ‘4-poster’ passive topical treatment device to apply acaricide for controlling ticks (Acari: Ixodidae) feeding on white-tailed deer. J Med Entomol 37(4):588–594
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rhoads CL, Bowman JL, Eyler B (2010) Home Range and Movement Rates of Female Exurban White-Tailed Deer. J Wildl Manag 74:987–994. https://doi.org/10.2193/2009-005
Roden-Reynolds P, Machtinger ET, Li AY, Mullinax JM(2020) Trapping white-tailed deer (Artiodactyla : Cervidae) in suburbia for study of tick–host interaction. J Insect Science, 20(6):1–12
Rooney T, Waller D (2003) Direct and indirect effects of white-tailed deer in forest ecosystems. For Ecol Manag 181:165–176. https://doi.org/10.1016/S0378-1127(03)00130-0
Rowcliffe JM, Carbone C, Kays R et al (2012) Bias in estimating animal travel distance: the effect of sampling frequency. Methods Ecol Evol 3:653–662. https://doi.org/10.1111/j.2041-210X.2012.00197.x
Severinghaus CW (1949) Tooth Development and Wear as Criteria of Age in White-Tailed Deer. J Wildl Manag 13:195–216. https://doi.org/10.2307/3796089
Simoneaux TN, Cohen BS, Cooney EA et al (2016) Fine-scale Movements of Adult Male White-tailed Deer in Northeastern Louisiana during the Hunting Season. J Southeast Assoc Fish Wildl Agencies 3:210–219
Stafford KC(2007) Tick management handbook: an integrated guide for homeowners, pest control operators, and public health officials for the prevention of tick-associated disease. Connecticut Agricultural Experiment Station. Revised Fall 2007. https://portal.ct.gov/-/media/CAES/DOCUMENTS/Publications/Bulletins/b1010pdf.pdf?la=en Accessed 02 January 2022
Stafford KC, Williams SC (2017) Deer-Targeted Methods: A Review of the Use of Topical Acaricides for the Control of Ticks on White-Tailed Deer. J Integr Pest Manag 8. https://doi.org/10.1093/jipm/pmx014
Stafford KC, Williams SC, Molaei G (2017) Integrated Pest Management in Controlling Ticks and Tick-Associated Diseases. J Integr Pest Manag 8. https://doi.org/10.1093/jipm/pmx018
Storm DJ, Nielsen CK, Schauber EM, Woolf A (2007) Space Use and Survival of White-Tailed Deer in an Exurban Landscape. J Wildl Manag 71:1170–1176. https://doi.org/10.2193/2006-388
Telford SR (2017) Deer Reduction Is a Cornerstone of Integrated Deer Tick Management. J Integr Pest Manag 8. https://doi.org/10.1093/jipm/pmx024
Telford SR, Mather TN, Moore SI et al (1988) Incompetence of deer as reservoirs of the Lyme disease spirochete. Am J Trop Med Hyg 39:105–109. https://doi.org/10.4269/ajtmh.1988.39.105
Tomberlin J(2007) Movement, Activity, and Habitat Use of Adult Male White-tailed Deer at Chesapeake Farms, Maryland. Thesis, North Carolina State University
United States Census Bureau (2019) American Community Survey 1-year estimates. http://censusreporter.org/profiles/05000US24027-howard-county-md/. Accessed 07 March 2021
Urbanek RE, Allen KR, Nielsen CK (2011) Urban and suburban deer management by state wildlife-conservation agencies. Wildl Soc Bull 35:310–315. https://doi.org/10.1002/wsb.37
Urbanek RE, Nielsen CK (2013) Influence of landscape factors on density of suburban white-tailed deer. Landsc Urban Plan 114:28–36. https://doi.org/10.1016/j.landurbplan.2013.02.006
Walter WD, Beringer J, Hansen LP et al (2011) Factors affecting space use overlap by white-tailed deer in an urban landscape. Int J Geogr Inf Sci 25:379–392. https://doi.org/10.1080/13658816.2010.524163
Walter WD, Evans TS, Stainbrook D et al (2018) Heterogeneity of a landscape influences size of home range in a North American cervid. Sci Rep 8:14667. https://doi.org/10.1038/s41598-018-32937-7
Walter WD, VerCauteren KC, Campa H et al (2009) Regional assessment on influence of landscape configuration and connectivity on range size of white-tailed deer. Landsc Ecol 24:1405–1420. https://doi.org/10.1007/s10980-009-9374-4
White A, Gaff H (2018) Review: Application of Tick Control Technologies for Blacklegged, Lone Star, and American Dog Ticks. J Integr Pest Manag 9. https://doi.org/10.1093/jipm/pmy006
Williams SC, Ward JS (2006) Exotic Seed Dispersal by White-tailed Deer in Southern Connecticut. Nat Areas J 26:383–390. https://doi.org/10.3375/0885-8608(2006)26[383:ESDBWD]2.0.CO;2
Wood SN (2004) Stable and Efficient Multiple Smoothing Parameter Estimation for Generalized Additive Models. J Am Stat Assoc 99:673–686. https://doi.org/10.1198/016214504000000980
Young I, Prematunge C, Pussegoda K, Corrin T, Waddell L (2021) Tick exposures and alpha-gal syndrome: A systematic review of the evidence. Ticks Tick Borne Dis May 12(3):101674. https://doi.org/10.1016/j.ttbdis.2021.101674
Acknowledgements
The authors thank Brenda Belensky, Phil Norman, and the rest of the deer project team at Howard County Department of Recreation and Parks for assistance with deer trapping and GPS collar recovery. We thank Laura Beimfohr, Yasmine Hentati, Grace Hummell, Carson Coriell, and Calvin Matson of the USDA Areawide Tick Project team for the dedicated work throughout the project. We also thank Brian Eyler and Maryland Department of Natural Resources for their guidance in the trapping process.
Funding
This study was supported by the Areawide Tick Management Project funds received from Office of National Programs, the United States Department of Agriculture (USDA) through a Non-Assistance Cooperative Agreement (# 58-8042-6-080) between the USDA Agricultural Research Service (ARS) and The University of Maryland. This article reports the results of research only. Mention of a proprietary product does not constitute an endorsement or a recommendation by the USDA for its use. The USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
Andrew Li and Jennifer Mullinax conceptualized the study. Patrick Roden-Reynolds, Cody Kent, and Jennifer Mullinax designed the statistical analysis. Patrick Roden-Reynolds and Cody Kent wrote the code for the analysis. Patrick Roden-Reynolds and Jennifer Mullinax wrote the manuscript. All authors participated in editing of the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
The authors have declared that no competing interests exist.
Ethics approval
The deer trapping protocol was approved by the Animal Care and Use Committee (IACUC approval #16–024) of the United States Department of Agriculture Beltsville Agricultural Research Center.
Consent to participate
Not Applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roden-Reynolds, P., Kent, C.M., Li, A.Y. et al. Patterns of white-tailed deer movements in suburban Maryland: implications for zoonotic disease mitigation. Urban Ecosyst 25, 1925–1938 (2022). https://doi.org/10.1007/s11252-022-01270-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11252-022-01270-3