Abstract
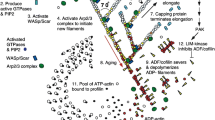
Actin cytoskeletal protrusions in crawling cells, or lamellipodia, exhibit various morphological properties such as two characteristic peaks in the distribution of filament orientation with respect to the leading edge. To understand these properties, using the dendritic nucleation model as a basis for cytoskeletal restructuring, a kinetic-population model with orientational-dependent branching (birth) and capping (death) is constructed and analyzed. Optimizing for growth yields a relation between the branch angle and filament orientation that explains the two characteristic peaks. The model also exhibits a subdominant population that allows for more accurate modeling of recent measurements of filamentous actin density along the leading edge of lamellipodia in keratocytes. Finally, we explore the relationship between orientational and spatial organization of filamentous actin in lamellipodia and address recent observations of a prevalence of overlapping filaments to branched filaments—a finding that is claimed to be in contradiction with the dendritic nucleation model.
Similar content being viewed by others
References
Bray D (2001) Cell movements: from molecules to motility. Garland, New York
Bock WJ, von Wahlert G (1965) Adaptation and the form-function complex. Evolution: Int J Organic Evol 19: 269–299
Brieher WM, Coughlin M, Mitchinson TJ (2004) Fascin-mediated propulsion of listeria monocytogenes independent of frequent nucleation by the arp2/3 complex. J Cell Biol 165: 233–242
Carlier M-F et al (2003) Actin-based motility as a self-organized system: mechanism and reconstitution in vitro. C. R. Biologies 326: 161–170
Carlsson AE (2004) Structure of autocatalytically branched actin solutions. Phys Rev Lett 92: 238102-1–238102-4
Carlsson AE (2005) The effect of branching on the critical concentration and avearage filament length. Biophys J 89: 130–140
Crow JF, Kimura M (1970) An introduction to population genetics theory. Harper & Row, New York
Fisher RA (1999) The genetical theory of natural selection. Oxford University Press, Oxford
Gopinathan A, Lee K-C, Schwarz JM, Liu AJ (2007) Branching, capping, and severing in dynamic actin structures. Phys Rev Lett 99: 058103-1–058103-4
Grimm HP, Verkhovsky AB, Mogilner A, Meister J-J (2003) Analysis of actin dynamics at the leading edge of crawling cells: implications for the shape of keratocyte lamellipodia. Eur Biophys J 32: 563–577
Hardik IE, Liu X, Ekambaram R (1991) Elastic stability of plates with varying rigidities. Comput Struct 38: 161–168
Holmes KC et al (1990) Atomic model for the actin filament. Nature 347: 44–49
Ichetovkin I, Grant W, Condeelis J (2002) Cofilin produces newly polymerized actin filaments that are preferred for dendritic nucleation by the arp2/3 complex. Curr Biol 12: 79–84
Kang H, Carlsson AE, Tang JX (2009) Kinetic overshoot in actin network assembly induced jointly by branching and capping proteins. Phys Rev E 80: 041913-1–041913-6
Keren K et al (2008) Mechanism of shape determination in motile cells. Nature 453: 475–480
Koestler SA et al (2008) Differentially oriented populations of actin filaments generated in lamellipodia collaborate in pushing and pausing at the cell front. Nat Cell Biol 10: 306–313
Lacayo CI et al (2007) Emergence of large-scale cell morphology and movement from local actin filament growth dynamics. PLoS Biol 5: 2035–2052
Lee K-C, Gopinathan A, Schwarz JM (2009) Modeling for formation of in vitro filopodia (arXiv:0909.2594)
Loisel TP et al (1999) Reconstitution of actin-based motility of listeria and shigella using pure proteins. Nature 401: 613–616
Maly IV, Borisy GG (2001) Self-organization of a propulsive actin network as an evolutionary process. Proc Natl Acad Sci USA 98: 483–518
May RC et al (1999) The arp2/3 complex is essential for the actin-based motility of listeria monocytogenes. Curr Biol 9: 759–762
Mogilner A (2009) Mathematics of cell motility: have we got its number. J Math Biol 58: 105–134
Mogilner A, Oster G (1996) Cell motility driven by actin polymerization. Biophys J 71: 3030–3045
Moore PB, Huxley HE, DeRosier DJ (1969) Three-dimensional reconstitution of f-actin, thin filaments and decorated filaments. J Mol Biol 50: 279–288
Mullins RD, Heuser JA, Pollard TD (1998) The interaction of arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA 95: 6181–6186
Odijk T (1998) Microfibrillar buckling within fibers under compression. J Chem Phys 108: 6923–6928
Parekh SH, Chaudhuri O, Theriot JA, Fletcher DA (2005) Loading history determines the velocity of actin-network growth. Nat Cell Biol 7: 1219–1223
Peskin CS, Odell GM, Oster GF (1993) Cellular motions and thermal fluctuations: The brownian ratchet. Biophys J 65: 316–324
Pollard TD, Blanchoin L, Mullins RD (2000) Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Ann Rev Biophys Biomol Struct 29: 545–576
Prass M, Jacobson K, Mogilner A, Radmacher M (2006) Direct measurement of the lamellipodial protrusive force in a migrating cell. J Cell Biol 174: 767–772
Rafelski SM, Theriot JA (2004) Crawling toward a unified model of cell mobility: spatial and temporal regulation of actin dynamics. Annu Rev Biochem 73: 209–239
Raucher D, Sheetz MP (2000) Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol 148: 127–136
Rubinstein B, Mogilner A (2005) The physics of filopodia protrusion. Biophys J 89: 782–795
Svitkina TM, Borisy GG (1999) Arp2/3 complex and actin depolymerizing factor/cofilin in dendritic organization and treadmilling of actin filament array in lamellipodia. J Cell Biol 145: 1009–1026
Urban E et al (2010) Electron tomography revels unbranched networks of actin filaments in lamellipodia. Nat Cell Biol 12: 429–435
Vignjevic D et al (2003) Formation of filapodia-like bundles in vitro from a dendritic network. J Cell Biol 160: 951–962
Weichsel J, Schwarz U (2010) Two competing orientation patterns explain experimentally observed anomalies in growing actin networks. Proc Natl Acad Sci USA 107: 6304–6309
Woodrum DT, Rich SA, Pollard TD (1975) Evidence for biased bidirectional polymerization of actin filaments using heavy meromyosin prepared by an imporved method. J Cell Biol 67: 231–237
Yang C, Hoelzle M, Disanza A, Scita G, Svitkina T (2009) Coordination of membrane and actin cytoskeleton dynamics during filopodia protrusion. PLoS ONE 4: e5678
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Quint, D.A., Schwarz, J.M. Optimal orientation in branched cytoskeletal networks. J. Math. Biol. 63, 735–755 (2011). https://doi.org/10.1007/s00285-010-0389-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-010-0389-x