Abstract
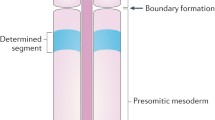
In vertebrate somitogenesis, “segmentation clock” genes (her in zebrafish, hes in mouse, and hairy in chick) show oscillation, synchronized over nearby cells through intercellular interaction. In zebrafish, neighboring cells interact by Delta-Notch signaling to realize synchronization. Under Delta-Notch, however, a cell with a high expression of the segmentation clock gene tends to suppress its expression in adjacent cells, which might produce spatial heterogeneity instead of synchronized oscillation. Here we studied the conditions under which pre-somitic mesoderm cells show synchronized oscillation of gene expression mathematically. We adopted a model that explicitly considers the kinetics of the mRNA and proteins of the segmentation clock gene and cell–cell interaction via Delta-Notch signaling. From statistical study of a model with randomly generated parameters, we revealed how the likelihood that the system generates stable synchronized oscillation depends on the rate of each reaction in the gene–protein kinetics.
Similar content being viewed by others
References
Baker RE, Schnell S, Maini PK (2006) A mathematical investigation of a Clock and Wavefront model for somitogenesis. J Math Biol 52: 458–482
Barrio M, Burrage K, Leier A, Tian T (2006) Oscillatory regulation of Hes1: Discrete stochastic delay modelling and simulation. PLoS Comput Biol 2(9): e117
Bessho Y, Miyoshi G, Sakata R, Kageyama R (2001) Hes7: a bHLH-type repressor gene regulated by Notch and expressed in the presomitic mesoderm. Genes Cells 6: 175–185
Cinquin O (2007) Repressor dimerization in the zebrafish somitogenesis clock. PLoS Comput Biol 3(2): e32
Collier JR, Monk NA, Maini PK, Lewis JH (1996) Pattern formation by lateral inhibition with feedback: a mathematical model of delta-notch intercellular signalling. J Theor Biol 183: 429–446
Collier JR, Mcinerney D, Schnell S, Maini PK, Gavaghan DJ, Houston P, Stern CD (2000) A cell cycle model for somitogenesis: mathematical formulation and numerical simulation. J Theor Biol 207: 305–316
Cooke J, Zeeman EC (1976) A clock and wavefront model for control of the number of repeated structures during animal morphogenesis. J Theor Biol 58: 455–476
Dubrulle J, Pourquie O (2002) From head to tail: links between the segmentation clock and antero-posterior patterning of the embryo. Curr Opin Genet Dev 12: 519–523
Giudicelli F, Ozbudak EM, Wright GJ, Lewis J (2007) Setting the tempo in development: an investigation of the zebrafish somite clock mechanism. PLoS Biol 5(6): e150
Goldbeter A, Pourquie O (2008) Modeling the segmentation clock as a network of coupled oscillations in the Notch, Wnt and FGF signaling pathways. J Theor Biol 252: 574–585
Hirata H, Yoshiura S, Ohtsuka T, Bessho Y, Harada T, Yoshikawa K, Kageyama R (2002) Oscillatory expression of the bHLH factor Hes1 regulated by a negative feedback loop. Science 298: 840–843
Holley SA, Geisler R, Nusslein-Volhard C (2000) Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev 14: 1678–1690
Holley SA, Julich D, Rauch GJ, Geisler R, Nusslein-Volhard C (2002) her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development 129: 1175–1183
Honda H, Tanemura M, Yoshida A (1990) Estimation of neuroblast numbers in insect neurogenesis using the lateral inhibition hypothesis of cell differentiation. Development 110: 1349–1352
Honda H, Tanemura M, Yoshida A (2000) Differentiation of wing epidermal scale cells in a butterfly under the lateral inhibition model—appearance of large cells in a polygonal pattern. Acta Biotheor 48: 121–136
Horikawa K, Ishimatsu K, Yoshimoto E, Kondo S, Takeda H (2006) Noise-resistant and synchronized oscillation of the segmentation clock. Nature 441: 719–723
Jaeger J, Goodwin BC (2002) Cellular oscillators in animal segmentation. In Silico Biol 2: 111–123
Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J (2000) Notch signalling and the synchronization of the somite segmentation clock. Nature 408: 475–479
Jouve C, Palmeirim I, Henrique D, Beckers J, Gossler A, Ish-Horowicz D, Pourquie O (2000) Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development 127: 1421–1429
Kaern M, Menzinger M, Hunding A (2000) Segmentation and somitogenesis derived from phase dynamics in growing oscillatory media. J Theor Biol 207: 473–493
Kerszberg M, Wolpert L (2000) A clock and trail model for somite formation, specialization and polarization. J Theor Biol 205: 505–510
Kobayashi R, Tero A, Nakagaki T (2006) Mathematical model for rhythmic protoplasmic movement in the true slime mold. J Math Biol 53: 273–286
Kuramoto Y (1984) Chemical oscillations, waves, and turbulence. Springer, Berlin
Kurosawa G, Iwasa Y (2002) Saturation of enzyme kinetics in circadian clock models. J Biol Rhythms 17: 568–577
Lewis J (2003) Autoinhibition with transcriptional delay: a simple mechanism for the zebrafish somitogenesis oscillator. Curr Biol 13: 1398–1408
Mara A, Schroeder J, Chalouni C, Holley SA (2007) Priming, initiation and synchronization of the segmentation clock by deltaD and deltaC. Nat Cell Biol 9: 523–530
Maroto M, Dale JK, Dequeant ML, Petit AC, Pourquie O (2005) Synchronised cycling gene oscillations in presomitic mesoderm cells require cell-cell contact. Int J Dev Biol 49: 309–315
Masamizu Y, Ohtsuka T, Takashima Y, Nagahara H, Takenaka Y, Yoshikawa K, Okamura H, Kageyama R (2006) Real-time imaging of the somite segmentation clock: revelation of unstable oscillators in the individual presomitic mesoderm cells. Proc Natl Acad Sci USA 103: 1313–1318
Meinhardt H (1982) Models of biological pattern formation. Academic, New York
Meinhardt H (1986) Somites in developing embryos. In: Bellairs R, Edie DA, Lash JW (eds) Nato ASI Series A, vol 118. Plenum Press, New York, pp 179–189
Niwa T (1995) An introduction to the theory of differential equations and dynamical systems. Yuseisya, Tokyo
Oates AC, Ho RK (2002) Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development 129: 2929–2946
Owen MR, Sherratt JA (1998) Mathematical modelling of juxtacrine cell signalling. Math Biosci 153: 125–150
Ozbudak EM, Lewis J (2008) Notch signalling synchronizes the zebrafish segmentation clock but is not needed to create somite boundaries. PLoS Genet 4(2): e15
Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O (1997) Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell 91: 639–648
Riedel-Kruse IH, Muller C, Oates AC (2007) Synchrony dynamics during initiation, failure, and rescue of the segmentation clock. Science 317: 1911–1915
Rodriguez-Gonzalez JG, Santillan M, Fowler AC, Mackey MC (2007) The segmentation clock in mice: interaction between the Wnt and Notch signalling pathways. J Theor Biol 248: 37–47
Rudge T, Burrage K (2008) Effects of intrinsic and extrinsic noise can accelerate juxtacrine pattern formation. Bull Math Biol 70: 971–991
Saga Y, Takeda H (2001) The making of the somite: molecular events in vertebrate segmentation. Nat Rev Genet 2: 835–845
Tanemura M, Honda H, Yoshida A (1991) Distribution of differentiated cells in a cell sheet under the lateral inhibition rule of differentiation. J Theor Biol 153: 287–300
Tiedemann HB, Schneltzer E, Zeiser S, Rubio-Aliaga I, Wurst W, Beckers J, Przemeck GKH, Hrabede Angelis M (2007) Cell-based simulation of dynamic expression patterns in the presomitic mesoderm. J Theor Biol 248: 120–129
Uriu K, Morishita Y, Iwasa Y (2009) Traveling wave formation in vertebrate segmentation. J Theor Biol 257: 385–396
Winfree AT (2000) The geometry of biological time. Springer, New York
Wu Z, Yamaguchi Y (2004) Input-dependent learning rule for the memory of spatiotemporal sequences in hippocampal network with theta phase precession. Biol Cybern 90: 113–124
Zeiser S, Muller J, Liebscher V (2007) Modeling the Hes1 oscillator. J Comput Biol 14: 984–1000
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uriu, K., Morishita, Y. & Iwasa, Y. Synchronized oscillation of the segmentation clock gene in vertebrate development. J. Math. Biol. 61, 207–229 (2010). https://doi.org/10.1007/s00285-009-0296-1
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00285-009-0296-1