Abstract
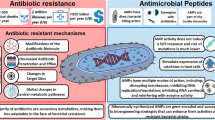
Antibiotic resistance is already widespread in the world, and it has become a great health problem. Therefore, comprehensive efforts are needed to minimize the resistance. The exploration of alternative therapies may offer a more targeted approach with less susceptibility to resistance. Even though antimicrobial peptides (AMPs) have been introduced as emerging antibiotic sources, they are not widely discussed in the literature. Since Neisseria infections show resistance to different types of antibiotics, the purpose of this review was to discuss the currently investigated AMPs with anti-Neisseria properties. In the present review, we provide an overview of 24 AMPs with in vitro anti-Neisseria properties.


Similar content being viewed by others
Data Availability
The data used in the present study is appropriately cited.
Abbreviations
- AMP:
-
Antimicrobial peptide
- WHO:
-
World Health Organization
- PID:
-
Pelvic inflammatory disease
- FPRL1:
-
Formyl peptide receptor-like 1
- TNFα:
-
Tumour necrosis factor alpha
- IL-6:
-
Interleukin 6
- LSA-5:
-
Liver stage antigen -5
- HD5:
-
Human α-defensin 5
- HNP-1:
-
Human neutrophil peptide-1
- HE2:
-
Human epididymis 2
- CCPs:
-
Cell-penetrating peptides
- TP10:
-
Transportan 10
References
Lenz JD, Dillard JP (2018) Pathogenesis of Neisseria gonorrhoeae and the host defense in ascending infections of human fallopian tube. Front Immunol 9:2710
Jen FE-C, Semchenko EA, Day CJ, Seib KL, Jennings MP (2019) The Neisseria gonorrhoeae methionine sulfoxide reductase (MsrA/B) is a surface exposed, immunogenic, vaccine candidate. Front Immunol 10:137
Unemo M, Shafer WM (2011) Antibiotic resistance in Neisseria gonorrhoeae: origin, evolution, and lessons learned for the future. Ann N Y Acad Sci 1230:E19
Vyse A, Wolter J, Chen J, Ng T, Soriano-Gabarro M (2011) Meningococcal disease in Asia: an under-recognized public health burden. Epidemiol Infect 139(7):967–985
Bala M, Sood S (2010) Cephalosporin resistance in Neisseria gonorrhoeae. J Glob Infect Dis 2(3):284
Unemo M, Nicholas RA (2012) Emergence of multidrug-resistant, extensively drug-resistant and untreatable gonorrhea. Future Microbiol 7(12):1401–1422
Girard MP, Preziosi M-P, Aguado M-T, Kieny MP (2006) A review of vaccine research and development: meningococcal disease. Vaccine 24(22):4692–4700
Savoia D, Guerrini R, Marzola E, Salvadori S (2008) Synthesis and antimicrobial activity of dermaseptin S1 analogues. Bioorg Med Chem 16(17):8205–8209
Cruz J, Ortiz C, Guzman F, Fernandez-Lafuente R, Torres R (2014) Antimicrobial peptides: promising compounds against pathogenic microorganisms. Curr Med Chem 21(20):2299–2321
Simonetti O, Cirioni O, Mocchegiani F, Cacciatore I, Silvestri C, Baldassarre L et al (2013) The efficacy of the quorum sensing inhibitor FS8 and tigecycline in preventing prosthesis biofilm in an animal model of staphylococcal infection. Int J Mol Sci 14(8):16321–16332
Zharkova MS, Orlov DS, Golubeva OY, Chakchir OB, Eliseev IE, Grinchuk TM et al (2019) Application of antimicrobial peptides of the innate immune system in combination with conventional antibiotics—a novel way to combat antibiotic resistance? Front Cell Infect Microbiol 9:128
Kościuczuk EM, Lisowski P, Jarczak J, Strzałkowska N, Jóźwik A, Horbańczuk J et al (2012) Cathelicidins: family of antimicrobial peptides. A review. Mol Biol Rep 39(12):10957–10970
Moncla B, Mietzner T, Hillier S (2012) In vitro activity of cationic peptides against Neisseria gonorrhoeae and vaginal Lactobacillus species: the effect of divalent cations. Adv Biosci Biotechnol, 3:249–255. https://doi.org/10.4236/abb.2012.33034
Bals R, Wang X, Zasloff M, Wilson JM (1998) The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface. Proc Natl Acad Sci USA 95(16):9541–9546
Bergman P, Johansson L, Asp V, Plant L, Gudmundsson GH, Jonsson AB et al (2005) Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell Microbiol 7(7):1009–1017
Schaller-Bals S, Schulze A, Bals R (2002) Increased levels of antimicrobial peptides in tracheal aspirates of newborn infants during infection. Am J Respir Crit Care Med 165(7):992–995
Zughaier SM, Svoboda P, Pohl J, Stephens DS, Shafer WM (2010) The human host defense peptide LL-37 interacts with Neisseria meningitidis capsular polysaccharides and inhibits inflammatory mediators release. PLoS ONE 5(10):e13627
Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J et al (2000) LL-37, the neutrophil granule–and epithelial cell–derived cathelicidin, utilizes formyl peptide receptor–like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192(7):1069–1074
Duplantier AJ, van Hoek ML (2013) The human cathelicidin antimicrobial peptide LL-37 as a potential treatment for polymicrobial infected wounds. Front Immunol 4:143
Cox DL, Sun Y, Liu H, Lehrer RI, Shafer WM (2003) Susceptibility of Treponema pallidum to host-derived antimicrobial peptides. Peptides 24(11):1741–1746
Xhindoli D, Pacor S, Benincasa M, Scocchi M, Gennaro R, Tossi A (2016) The human cathelicidin LL-37—a pore-forming antibacterial peptide and host-cell modulator. Biochimica Biophys Acta (BBA)—Biomembr 1858(3):546–566
Nagaoka I, Hirota S, Niyonsaba F, Hirata M, Adachi Y, Tamura H et al (2002) Augmentation of the lipopolysaccharide-neutralizing activities of human cathelicidin CAP18/LL-37-derived antimicrobial peptides by replacement with hydrophobic and cationic amino acid residues. Clin Diagn Lab Immunol 9(5):972–982
Kandler K, Shaykhiev R, Kleemann P, Klescz F, Lohoff M, Vogelmeier C et al (2006) The anti-microbial peptide LL-37 inhibits the activation of dendritic cells by TLR ligands. Int Immunol 18(12):1729–1736
Fukumoto K, Nagaoka I, Yamataka A, Kobayashi H, Yanai T, Kato Y et al (2005) Effect of antibacterial cathelicidin peptide CAP18/LL-37 on sepsis in neonatal rats. Pediatr Surg Int 21(1):20–24
Sato H, Feix JB (2006) Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochimica Biophys Acta (BBA)—Biomembr 1758(9):1245–1256
Kai-Larsen Y, Agerberth B (2008) The role of the multifunctional peptide LL-37 in host defense. Front Biosci 13(10):3760–3767
Kiattiburut W, Zhi R, Lee SG, Foo AC, Hickling DR, Keillor JW et al (2018) Antimicrobial peptide LL-37 and its truncated forms, GI-20 and GF-17, exert spermicidal effects and microbicidal activity against Neisseria gonorrhoeae. Hum Reprod 33(12):2175–2183
Jones A, Geörg M, Maudsdotter L, Jonsson A-B (2009) Endotoxin, capsule, and bacterial attachment contribute to Neisseria meningitidis resistance to the human antimicrobial peptide LL-37. J Bacteriol 191(12):3861–3868
Moncla BJ, Pryke K, Rohan LC, Graebing PW (2011) Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv Biosci Biotechnol (Print) 2(6):404
Nakamura T, Furunaka H, Miyata T, Tokunaga F, Muta T, Iwanaga S et al (1988) Tachyplesin, a class of antimicrobial peptide from the hemocytes of the horseshoe crab (Tachypleus tridentatus). Isolation and chemical structure. J Biol Chem 263(32):16709–16713
Shafer W, Qu X-D, Waring A, Lehrer R (1998) Modulation of Neisseria gonorrhoeae susceptibility to vertebrate antibacterial peptides due to a member of the resistance/nodulation/division efflux pump family. Proc Natl Acad Sci USA 95(4):1829–1833
Hancock RE, Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16(2):82–88
Kushibiki T, Kamiya M, Aizawa T, Kumaki Y, Kikukawa T, Mizuguchi M et al (2014) Interaction between tachyplesin I, an antimicrobial peptide derived from horseshoe crab, and lipopolysaccharide. Biochim Biophys Acta (BBA)—Proteins Proteomics 1844(3):527–534
Edwards IA, Elliott AG, Kavanagh AM, Blaskovich MA, Cooper MA (2017) Structure-activity and− toxicity relationships of the antimicrobial peptide tachyplesin-1. ACS Infect Dis 3(12):917–926
Qu X-D, Harwig S, Oren A, Shafer WM, Lehrer RI (1996) Susceptibility of Neisseria gonorrhoeae to protegrins. Infect Immun 64(4):1240–1245
Steinberg DA, Hurst MA, Fujii CA, Kung A, Ho J, Cheng F et al (1997) Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob Agents Chemother 41(8):1738–1742
Qu X-D, Harwig S, Shafer WM, Lehrer RI (1997) Protegrin structure and activity against Neisseria gonorrhoeae. Infect Immun 65(2):636–639
Lee SB, Li B, Jin S, Daniell H (2011) Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J 9(1):100–115
Kokryakov VN, Harwig SS, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV et al (1993) Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett 327(2):231–236
Tamamura H, Murakami T, Horiuchi S, Sugihara K, Otaka A, Takada W et al (1995) Synthesis of protegrin-related peptides and their antibacterial and anti-human immunodeficiency virus activity. Chem Pharm Bull 43(5):853–858
Hagman KE, Lucas CE, Balthazar JT, Snyder L, Nilles M, Judd RC et al (1997) The MtrD protein of Neisseria gonorrhoeae is a member of the resistance/nodulation/division protein family constituting part of an efflux system. Microbiology 143(7):2117–2125
Arpornsuwan T, Buasakul B, Jaresitthikunchai J, Roytrakul S (2014) Potent and rapid antigonococcal activity of the venom peptide BmKn2 and its derivatives against different Maldi biotype of multidrug-resistant Neisseria gonorrhoeae. Peptides 53:315–320
Epand RM, Vogel HJ (1999) Diversity of antimicrobial peptides and their mechanisms of action. Biochimica Biophys Acta (BBA)—Biomembr 1462(1–2):11–28
Sikora AE, Mills RH, Weber JV, Hamza A, Passow BW, Romaine A et al (2017) Peptide inhibitors targeting the Neisseria gonorrhoeae pivotal anaerobic respiration factor AniA. Antimicrob Agents Chemother 61(8):e00186-e217
Mellies J, Jose J, Meyer T (1997) The Neisseria gonorrhoeae gene aniA encodes an inducible nitrite reductase. Mol Gen Genet MGG 256(5):525–532
Kastin A (2013) Handbook of biologically active peptides. Academic press, Cambridge
Wu Z, Prahl A, Powell R, Ericksen B, Lubkowski J, Lu W (2003) From pro defensins to defensins: synthesis and characterization of human neutrophil pro α-defensin-1 and its mature domain. J Pept Res 62(2):53–62
Ghosh D, Porter E, Shen B, Lee SK, Wilk D, Drazba J et al (2002) Paneth cell trypsin is the processing enzyme for human defensin-5. Nat Immunol 3(6):583–590
Jones DE, Bevins CL (1992) Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem 267(32):23216–23225
Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip K-P et al (1998) Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol 152(5):1247
Porter E, Yang H, Yavagal S, Preza GC, Murillo O, Lima H et al (2005) Distinct defensin profiles in Neisseria gonorrhoeae and Chlamydia trachomatis urethritis reveal novel epithelial cell-neutrophil interactions. Infect Immun 73(8):4823–4833
Scocchi M, Skerlavaj B, Romeo D, Gennaro R (1992) Proteolytic cleavage by neutrophil elastase converts inactive storage proforms to antibacterial bactenecins. Eur J Biochem 209(2):589–595
Aarbiou J, Ertmann M, van Wetering S, van Noort P, Rook D, Rabe KF et al (2002) Human neutrophil defensins induce lung epithelial cell proliferation in vitro. J Leukoc Biol 72(1):167–174
Hook EW, Handsfield HH (1999) Gonococcal infections in the adult. In: Holmes KK, Mardh PA, Sparling PF, et al (eds) Sexually transmitted diseases, 3rd edn. McGraw-Hill, New York
Mor A, Nicolas P (1994) Isolation and structure of novel defensive peptides from frog skin. Eur J Biochem 219(1–2):145–154
Mor A, Van Huong N, Delfour A, Migliore-Samour D, Nicolas P (1991) Isolation, amino acid sequence and synthesis of dermaseptin, a novel antimicrobial peptide of amphibian skin. Biochemistry 30(36):8824–8830
Zairi A, Tangy F, Ducos-Galand M, Alonso J-M, Hani K (2007) Susceptibility of Neisseria gonorrhoeae to antimicrobial peptides from amphibian skin, dermaseptin, and derivatives. Diagn Microbiol Infect Dis 57(3):319–324
Navon-Venezia S, Feder R, Gaidukov L, Carmeli Y, Mor A (2002) Antibacterial properties of dermaseptin S4 derivatives with in vivo activity. Antimicrob Agents Chemother 46(3):689–694
Lorin C, Saidi H, Belaid A, Zairi A, Baleux F, Hocini H et al (2005) The antimicrobial peptide dermaseptin S4 inhibits HIV-1 infectivity in vitro. Virology 334(2):264–275
Belaid A, Aouni M, Khelifa R, Trabelsi A, Jemmali M, Hani K (2002) In vitro antiviral activity of dermaseptins against herpes simplex virus type 1. J Med Virol 66(2):229–234
Silva O, Ferreira E, Pato MV, Caniça M, Gomes ET (2002) In vitro anti-Neisseria gonorrhoeae activity of Terminalia macroptera leaves. FEMS Microbiol Lett 217(2):271–274
Gaidukov L, Fish A, Mor A (2003) Analysis of membrane-binding properties of dermaseptin analogues: relationships between binding and cytotoxicity. Biochemistry 42(44):12866–12874
Yenugu S, Narmadha G (2010) The human male reproductive tract antimicrobial peptides of the HE2 family exhibit potent synergy with standard antibiotics. J Pept Sci 16(7):337–341
Yenugu S, Hamil KG, French FS, Hall SH (2004) Antimicrobial actions of the human epididymis 2 (HE2) protein isoforms, HE2alpha, HE2beta1 and HE2beta2. Reprod Biol Endocrinol 2(1):61
Liao M, Ruddock P, Rizvi A, Hall S, French F, Dillon J (2005) Cationic peptide of the male reproductive tract, HE2a, displays antimicrobial activity against Neisseria gonorrhoeae, Staphylococcus aureus and Enterococcus faecalis. J Antimicrob Chemother 56(5):957–961. https://doi.org/10.1093/jac/dki350
Yenugu S, Hamil KG, Birse CE, Ruben SM, French FS, Hall SH (2003) Antibacterial properties of the sperm-binding proteins and peptides of human epididymis 2 (HE2) family; salt sensitivity, structural dependence and their interaction with outer and cytoplasmic membranes of Escherichia coli. Biochem J 372(2):473–483
Parada JL, Caron CR, Medeiros ABP, Soccol CR (2007) Bacteriocins from lactic acid bacteria: purification, properties and use as biopreservatives. Braz Arch Biol Technol 50(3):512–542
Yang S-C, Lin C-H, Sung CT, Fang J-Y (2014) Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol 5:241
Kaletta C, Entian K-D (1989) Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol 171(3):1597–1601
Gómez GC, Castillo JCQ, Pérez JCA, Montoya JEZ (2012) Ethanolic extract from leaves of Bixaorellana L.: a potential natural food preservative. Interciencia 37(7):547–551
Rodriguez J (1996) Review: antimicrobial spectrum, structure, properties and mode of action of nisin, a bacteriocin produced by Lactococcus lactis. Food Sci Technol Int 2(2):61–68
Field D, Begley M, O’Connor PM, Daly KM, Hugenholtz F, Cotter PD et al (2012) Bioengineered nisin A derivatives with enhanced activity against both gram positive and gram negative pathogens. PLoS ONE 7(10):e46884
Mota-Meira M, Lapointe G, Lacroix C, Lavoie MC (2000) MICs of mutacin B-Ny266, nisin A, vancomycin, and oxacillin against bacterial pathogens. Antimicrob Agents Chemother 44(1):24–29
Madanchi H, Sardari S (2019) The role of antimicrobial peptides in the prevention of sexually transmitted infection (STI). MOJ Womens Health 8(2):192–194
Morency H, Trahan L, Lavoie M (1995) Preliminary grouping of mutacins. Can J Microbiol 41(9):826–831
Mota-Meira M, Morency H, Lavoie MC (2005) In vivo activity of mutacin B-Ny266. J Antimicrob Chemother 56(5):869–871
Mattick A, Hirsch A (1947) Further observations on an inhibitory substance (nisin) from lactic streptococci. Lancet 5:5–8
Parrot M, Caufield P, Lavoie M (1990) Preliminary characterization of four bacteriocins from Streptococcus mutans. Can J Microbiol 36(2):123–130
Mota-Meira M, Lacroix C, LaPointe G, Lavoie MC (1997) Purification and structure of mutacin B-Ny266: a new lantibiotic produced by Streptococcus mutans. FEBS Lett 410(2–3):275–279
Fonseca SB, Pereira MP, Kelley SO (2009) Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv Drug Deliv Rev 61(11):953–964
Uchida T, Kanazawa T, Kawai M, Takashima Y, Okada H (2011) Therapeutic effects on atopic dermatitis by anti-RelA short interfering RNA combined with functional peptides Tat and AT1002. J Pharmacol Exp Ther 338(2):443–450
Palm C, Netzereab S, Hällbrink M (2006) Quantitatively determined uptake of cell-penetrating peptides in non-mammalian cells with an evaluation of degradation and antimicrobial effects. Peptides 27(7):1710–1716
Splith K, Neundorf I (2011) Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J 40(4):387–397
Soomets U, Lindgren M, Gallet X, Hällbrink M, Elmquist A, Balaspiri L et al (2000) Deletion analogues of transportan. Biochim Biophys Acta (BBA)—Biomembr 1467(1):165–176
Derossi D, Chassaing G, Prochiantz A (1998) Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol 8(2):84–87
Yandek LE, Pokorny A, Florén A, Knoelke K, Langel Ü, Almeida PF (2007) Mechanism of the cell-penetrating peptide transportan 10 permeation of lipid bilayers. Biophys J 92(7):2434–2444
Takeshima K, Chikushi A, Lee K-K, Yonehara S, Matsuzaki K (2003) Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J Biol Chem 278(2):1310–1315
Eriksson OS, Geörg M, Sjölinder H, Sillard R, Lindberg S, Langel Ü et al (2013) Identification of cell-penetrating peptides that are bactericidal to Neisseria meningitidis and prevent inflammatory responses upon infection. Antimicrob Agents Chemother 57(8):3704–3712
Kozlowski LP (2016) IPC–isoelectric point calculator. Biol Direct 11(1):1–16
Funding
The review was conducted without funding.
Author information
Authors and Affiliations
Contributions
Idea: PA and AN; Performing a search of the literature: PA; Writing—original draft preparation: PA; Writing—review and editing: PA, MY, MF, MA, NL and AM; Supervision: KG and MHN.
Corresponding authors
Ethics declarations
Conflict of interest
Manuscript title: Review of antimicrobial peptides with anti‐Neisseria activity. The authors have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Askari, P., Yousefi, M., Foadoddini, M. et al. Antimicrobial Peptides as a Promising Therapeutic Strategy for Neisseria Infections. Curr Microbiol 79, 102 (2022). https://doi.org/10.1007/s00284-022-02767-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00284-022-02767-y