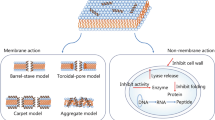
Abstract
Antibiotic resistance is an imminent threat to the effective treatment of bacterial infections, and alternative antibiotic strategies are urgently required. The golden epoch of antibiotics is coming to an end, and the development of new therapeutic agents to combat bacterial infections should be prioritized. This article will review the potential of antimicrobial peptides (AMPs) to combat the threat of antimicrobial resistance. The modern-day antimicrobial resistance dilemma is briefly discussed followed by a review of the potential of AMPs to be used alone or in combination with current antibiotics in order to enhance antibacterial properties of antibiotics while also potentially combatting resistance. This article reiterates that many AMPs exhibit direct microbial killing activity and also play an integral role in the innate immune system. These properties make AMPs attractive alternative antimicrobial agents. Furthermore, AMPs are promising candidates to be used as adjuvants in combination with current antibiotics in order to combat antibiotic resistance. Combinations of AMPs and antibiotics are less likely to develop resistance or transmit cross-resistance. The further identification and therapeutic development of AMPs and antibiotic-AMP combinations are strongly recommended.

Similar content being viewed by others
References
O’Neill J (2016) The review on antimicrobial resistance. Tackling drug-resistant infections globally: final report and recommendations https://amr-revieworg/sites/default/files/160518_Final%20paper_with%20coverpdf Accessed 16 January 2017
Brundage JF, Shanks GD (2008) Deaths from bacterial pneumonia during 1918-19 influenza pandemic. Emerg Infect Dis 14(8):1193–1199. https://doi.org/10.3201/eid1408.071313
Morens DM, Taubenberger JK, Fauci AS (2008) Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 198(7):962–970. https://doi.org/10.1086/591708
Garau J (2002) Treatment of drug-resistant pneumococcal pneumonia. Lancet Infect Dis 2(7):404–415. https://doi.org/10.1016/S1473-3099(02)00316-X
Ho PL, Cheng VC, Chu CM (2009) Antibiotic resistance in community-acquired pneumonia caused by Streptococcus pneumoniae, methicillin-resistant Staphylococcus aureus, and Acinetobacter baumannii. Chest 136(4):1119–1127. https://doi.org/10.1378/chest.09-0285
Buckle GC, Walker CLF, Black RE (2012) Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Glob Health 2(1):010401
Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, Kingsley RA, Thomson NR, Keane JA, Weill FX, Edwards DJ, Hawkey J, Harris SR, Mather AE, Cain AK, Hadfield J, Hart PJ, Thieu NTV, Klemm EJ, Glinos DA, Breiman RF, Watson CH, Kariuki S, Gordon MA, Heyderman RS, Okoro C, Jacobs J, Lunguya O, Edmunds WJ, Msefula C, Chabalgoity JA, Kama M, Jenkins K, Dutta S, Marks F, Campos J, Thompson C, Obaro S, MacLennan CA, Dolecek C, Keddy KH, Smith AM, Parry CM, Karkey A, Mulholland EK, Campbell JI, Dongol S, Basnyat B, Dufour M, Bandaranayake D, Naseri TT, Singh SP, Hatta M, Newton P, Onsare RS, Isaia L, Dance D, Davong V, Thwaites G, Wijedoru L, Crump JA, de Pinna E, Nair S, Nilles EJ, Thanh DP, Turner P, Soeng S, Valcanis M, Powling J, Dimovski K, Hogg G, Farrar J, Holt KE, Dougan G (2015) Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47(6):632–639. https://doi.org/10.1038/ng.3281
Yan M, Li X, Liao Q, Li F, Zhang J, Kan B (2016) The emergence and outbreak of multidrug-resistant typhoid fever in China. Emerg Microbes Infect 5(6):e62. https://doi.org/10.1038/emi.2016.62
Nataro JP, Kaper JB (1998) Diarrheagenic Escherichia coli. Clin Microbiol Rev 11(1):142–201
Lim JY, Hong JB, Sheng H, Shringi S, Kaul R, Besser TE, Hovde CJ (2010) Phenotypic diversity of Escherichia coli O157:H7 strains associated with the plasmid O157. J Microbiol 48(3):347–357. https://doi.org/10.1007/s12275-010-9228-4
World Health Organization (2017) Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics http://wwwwhoint/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ Accessed 12 October 2017
Centres for Disease Control and Prevention (2013) Antibiotic resistance threats in the United States. https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf Accessed 17 July 2017
Chikindas ML, Weeks R, Drider D, Chistyakov VA, Dicks LM (2018) Functions and emerging applications of bacteriocins. Curr Opin Biotechnol 49:23–28. https://doi.org/10.1016/j.copbio.2017.07.011
Fox JL (2013) Antimicrobial peptides stage a comeback. Nat Biotechnol 31(5):379–382. https://doi.org/10.1038/nbt.2572
Hancock RE, Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol 16(2):82–88
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24(12):1551–1557. https://doi.org/10.1038/nbt1267
Fleming A (1929) On the antibacterial action of cultures of a penicillium, with special reference to their use in the isolation of B. influenzae. Br J Exp Pathol 10(3):226–236
Read AF, Woods RJ (2014) Antibiotic resistance management. Evol Med Public Health 2014(1):147. https://doi.org/10.1093/emph/eou024
Cirz RT, Chin JK, Andes DR, de Crecy-Lagard V, Craig WA, Romesberg FE (2005) Inhibition of mutation and combating the evolution of antibiotic resistance. PLoS Biol 3(6):e176. https://doi.org/10.1371/journal.pbio.0030176
Munita JM, Arias CA (2016) Mechanisms of antibiotic resistance. Microbiol Spectr 4(2). https://doi.org/10.1128/microbiolspec.VMBF-0016-2015
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433. https://doi.org/10.1128/MMBR.00016-10
Blumberg HM, Rimland D, Carroll DJ, Terry P, Wachsmuth IK (1991) Rapid development of ciprofloxacin resistance in methicillin-susceptible and -resistant Staphylococcus aureus. J Infect Dis 163(6):1279–1285
Appelbaum PC (2007) Reduced glycopeptide susceptibility in methicillin-resistant Staphylococcus aureus (MRSA). Int J Antimicrob Agents 30(5):398–408. https://doi.org/10.1016/j.ijantimicag.2007.07.011
Faron ML, Ledeboer NA, Buchan BW (2016) Resistance mechanisms, epidemiology, and approaches to screening for vancomycin-resistant enterococcus in the health care setting. J Clin Microbiol 54(10):2436–2447. https://doi.org/10.1128/JCM.00211-16
Chen L, Todd R, Kiehlbauch J, Walters M, Kallen A (2017) Notes from the field: pan-resistant New Delhi metallo-beta-lactamase-producing Klebsiella pneumoniae. MMWR Morb Mortal Wkly Rep 66(1):33. https://doi.org/10.15585/mmwr.mm6601a7
Brooks B (2016) Studies find ‘super bacteria’ in Rio's Olympic venues, top beaches. Reuters http://www.reuters.com/article/us-olympics-rio-superbacteria-exclusive-idUSKCN0YW2E8 Accessed 23 August 2017
Dubos RJ (1939) Studies on a bactericidal agent extracted from a soil Bacillus : I. preparation of the agent. Its Activity in Vitro J Exp Med 70(1):1–10
Wang G, Li X, Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res 44(D1):D1087–D1093. https://doi.org/10.1093/nar/gkv1278
Fleming A (1922) On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond B 93:306–317. https://doi.org/10.1098/rspb.1922.0023
Vocadlo DJ, Davies GJ, Laine R, Withers SG (2001) Catalysis by hen egg-white lysozyme proceeds via a covalent intermediate. Nature 412:835–838. https://doi.org/10.1038/35090602
Elmogy M, Bassal TT, Yousef HA, Dorrah MA, Mohamed AA, Duvic B (2015) Isolation, characterization, kinetics, and enzymatic and nonenzymatic microbicidal activities of a novel c-type lysozyme from plasma of Schistocerca gregaria (Orthoptera: Acrididae). J Insect Sci 15(1):57. https://doi.org/10.1093/jisesa/iev038
Gallo RL (2013) The birth of innate immunity. Exp Dermatol 22(8):517. https://doi.org/10.1111/exd.12197
Van Epps HL (2006) René Dubos: unearthing antibiotics. J Exp Med 203(2):259–259. https://doi.org/10.1084/jem.2032fta
Wang G, Li X, Wang Z (2017) The antimicrobial peptide database. http://apsunmcedu/AP/mainphp Accessed 20 March 2017
Jenssen H, Hamill P, Hancock RE (2006) Peptide antimicrobial agents. Clin Microbiol Rev 19(3):491–511. https://doi.org/10.1128/CMR.00056-05
Schweizer F (2009) Cationic amphiphilic peptides with cancer-selective toxicity. Eur J Pharmacol 625(1–3):190–194. https://doi.org/10.1016/j.ejphar.2009.08.043
Lewies A, Wentzel JF, Miller HC, Du Plessis LH (2018) The antimicrobial peptide nisin Z induces selective toxicity and apoptotic cell death in cultured melanoma cells. Biochimie 144:28–40. https://doi.org/10.1016/j.biochi.2017.10.009
Gordon YJ, Romanowski EG, McDermott AM (2005) A review of antimicrobial peptides and their therapeutic potential as anti-infective drugs. Curr Eye Res 30(7):505–515. https://doi.org/10.1080/02713680590968637
Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL (2006) Colistin: the re-emerging antibiotic for multidrug-resistant Gram-negative bacterial infections. Lancet Infect Dis 6(9):589–601. https://doi.org/10.1016/S1473-3099(06)70580-1
Steenbergen JN, Alder J, Thorne GM, Tally FP (2005) Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections. J Antimicrob Chemother 55(3):283–288. https://doi.org/10.1093/jac/dkh546
Tran TT, Munita JM, Arias CA (2015) Mechanisms of drug resistance: daptomycin resistance. Ann N Y Acad Sci 1354(1):32–53. https://doi.org/10.1111/nyas.12948
Hancock RE, Diamond G (2000) The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol 8(9):402–410
Midorikawa K, Ouhara K, Komatsuzawa H, Kawai T, Yamada S, Fujiwara T, Yamazaki K, Sayama K, Taubman MA, Kurihara H, Hashimoto K, Sugai M (2003) Staphylococcus aureus susceptibility to innate antimicrobial peptides, beta-defensins and CAP18, expressed by human keratinocytes. Infect Immun 71(7):3730–3739
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3(10):777–788. https://doi.org/10.1038/nrmicro1273
Hassan M, Kjos M, Nes IF, Diep DB, Lotfipour F (2014) Antimicrobial peptides from prokaryotes. In: Phoenix DA, Harris F, Dennison SR (eds) Novel antimicrobial agents and strategies. Wiley, New York. https://doi.org/10.1002/9783527676132.ch5
McAuliffe O, Ross RP, Hill C (2001) Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev 25(3):285–308
Shin JM, Gwak JW, Kamarajan P, Fenno JC, Rickard AH, Kapila YL (2016) Biomedical applications of nisin. J Appl Microbiol 120(6):1449–1465. https://doi.org/10.1111/jam.13033
Jones E, Salin V, Williams GW (2005) Nisin and the market for commercial bacteriocins. TAMRC consumer and product research report no. CP-01-05. https://ageconsearch.umn.edu/bitstream/90779/2/CP%2001%2005%20Nisin%20Report.pdf Accessed 18 August 2015
Gill SR, Fouts DE, Archer GL, Mongodin EF, DeBoy RT, Ravel J, Paulsen IT, Kolonay JF, Brinkac L, Beanan M, Dodson RJ, Daugherty SC, Madupu R, Angiuoli SV, Durkin AS, Haft DH, Vamathevan J, Khouri H, Utterback T, Lee C, Dimitrov G, Jiang L, Qin H, Weidman J, Tran K, Kang K, Hance IR, Nelson KE, Fraser CM (2005) Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J Bacteriol 187(7):2426–2438. https://doi.org/10.1128/JB.187.7.2426-2438.2005
Oliveira M, Bexiga R, Nunes SF, Carneiro C, Cavaco LM, Bernardo F, Vilela CL (2006) Biofilm-forming ability profiling of Staphylococcus aureus and Staphylococcus epidermidis mastitis isolates. Vet Microbiol 118(1–2):133–140. https://doi.org/10.1016/j.vetmic.2006.07.008
Cao LT, Wu JQ, Xie F, Hu SH, Mo Y (2007) Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci 90(8):3980–3985. https://doi.org/10.3168/jds.2007-0153
Wu J, Hu S, Cao L (2007) Therapeutic effect of nisin Z on subclinical mastitis in lactating cows. Antimicrob Agents Chemother 51(9):3131–3135. https://doi.org/10.1128/AAC.00629-07
Pieterse R, Todorov SD (2010) Bacteriocins - exploring alternatives to antibiotics in mastitis treatment. Braz J Microbiol 41(3):542–562. https://doi.org/10.1590/S1517-83822010000300003
Goldstein BP, Wei J, Greenberg K, Novick R (1998) Activity of nisin against Streptococcus pneumoniae, in vitro, and in a mouse infection model. J Antimicrob Chemother 42(2):277–278
Brumfitt W, Salton MR, Hamilton-Miller JM (2002) Nisin, alone and combined with peptidoglycan-modulating antibiotics: activity against methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci. J Antimicrob Chemother 50(5):731–734
Dosler S, Gerceker AA (2011) In vitro activities of nisin alone or in combination with vancomycin and ciprofloxacin against methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. Chemotherapy 57(6):511–516. https://doi.org/10.1159/000335598
Bartoloni A, Mantella A, Goldstein BP, Dei R, Benedetti M, Sbaragli S, Paradisi F (2004) In-vitro activity of nisin against clinical isolates of Clostridium difficile. J Chemother 16(2):119–121. https://doi.org/10.1179/joc.2004.16.2.119
Wright GD (2016) Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol 24(11):862–871. https://doi.org/10.1016/j.tim.2016.06.009
Radek K, Gallo R (2007) Antimicrobial peptides: natural effectors of the innate immune system. Semin Immunopathol 29(1):27–43
Rishi P, Preet S, Bharrhan S, Verma I (2011) In vitro and in vivo synergistic effects of cryptdin 2 and ampicillin against Salmonella. Antimicrob Agents Chemother 55(9):4176–4182. https://doi.org/10.1128/AAC.00273-11
Choi H, Lee DG (2012) Synergistic effect of antimicrobial peptide arenicin-1 in combination with antibiotics against pathogenic bacteria. Res Microbiol 163(6–7):479–486. https://doi.org/10.1016/j.resmic.2012.06.001
Naghmouchi K, Le Lay C, Baah J, Drider D (2012) Antibiotic and antimicrobial peptide combinations: synergistic inhibition of Pseudomonas fluorescens and antibiotic-resistant variants. Res Microbiol 163(2):101–108. https://doi.org/10.1016/j.resmic.2011.11.002
Mataraci E, Dosler S (2012) In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob Agents Chemother 56(12):6366–6371. https://doi.org/10.1128/AAC.01180-12
Dosler S, Gerceker AA (2012) In vitro activities of antimicrobial cationic peptides; melittin and nisin, alone or in combination with antibiotics against Gram-positive bacteria. J Chemother 24(3):137–143. https://doi.org/10.1179/1973947812Y.0000000007
Zhang Y, Liu Y, Sun Y, Liu Q, Wang X, Li Z, Hao J (2014) In vitro synergistic activities of antimicrobial peptide brevinin-2CE with five kinds of antibiotics against multidrug-resistant clinical isolates. Curr Microbiol 68(6):685–692. https://doi.org/10.1007/s00284-014-0529-4
Nuding S, Frasch T, Schaller M, Stange EF, Zabel LT (2014) Synergistic effects of antimicrobial peptides and antibiotics against Clostridium difficile. Antimicrob Agents Chemother 58(10):5719–5725. https://doi.org/10.1128/AAC.02542-14
Rishi P, Preet Singh A, Garg N, Rishi M (2014) Evaluation of nisin-beta-lactam antibiotics against clinical strains of Salmonella enterica serovar Typhi. J Antibiot (Tokyo) 67(12):807–811. https://doi.org/10.1038/ja.2014.75
Lin L, Nonejuie P, Munguia J, Hollands A, Olson J, Dam Q, Kumaraswamy M, Rivera H Jr, Corriden R, Rohde M, Hensler ME, Burkart MD, Pogliano J, Sakoulas G, Nizet V (2015) Azithromycin synergizes with cationic antimicrobial peptides to exert bactericidal and therapeutic activity against highly multidrug-resistant Gram-negative bacterial pathogens. EBioMedicine 2(7):690–698. https://doi.org/10.1016/j.ebiom.2015.05.021
Lewies A, Wentzel JF, Jordaan A, Bezuidenhout C, Du Plessis LH (2017) Interactions of the antimicrobial peptide nisin Z with conventional antibiotics and the use of nanostructured lipid carriers to enhance antimicrobial activity. Int J Pharm 526(1–2):244–253. https://doi.org/10.1016/j.ijpharm.2017.04.071
Lewies A, Wentzel JF, Jacobs G, Du Plessis LH (2015) The potential use of natural and structural analogues of antimicrobial peptides in the fight against neglected tropical diseases. Molecules 20(8):15392–15433. https://doi.org/10.3390/molecules200815392
Wu G, Ding J, Li H, Li L, Zhao R, Shen Z, Fan X, Xi T (2008) Effects of cations and pH on antimicrobial activity of thanatin and s-thanatin against Escherichia coli ATCC25922 and B. subtilis ATCC 21332. Curr Microbiol 57(6):552–557. https://doi.org/10.1007/s00284-008-9241-6
Cohen J (2002) The immunopathogenesis of sepsis. Nature 420(6917):885–891. https://doi.org/10.1038/nature01326
Mueller M, Lindner B, Dedrick R, Schromm AB, Seydel U (2005) Endotoxin: physical requirements for cell activation. J Endotoxin Res 11(5):299–303
Mangoni ML, Epand RF, Rosenfeld Y, Peleg A, Barra D, Epand RM, Shai Y (2008) Lipopolysaccharide, a key molecule involved in the synergism between temporins in inhibiting bacterial growth and in endotoxin neutralization. J Biol Chem 283(34):22907–22917. https://doi.org/10.1074/jbc.M800495200
Rosenfeld Y, Papo N, Shai Y (2006) Endotoxin (lipopolysaccharide) neutralization by innate immunity host-defense peptides. Peptide properties and plausible modes of action. J Biol Chem 281(3):1636–1643. https://doi.org/10.1074/jbc.M504327200
Mookherjee N, Brown KL, Bowdish DM, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, Powers JP, Bryan J, Brinkman FS, Hancock RE (2006) Modulation of the TLR-mediated inflammatory response by the endogenous human host defense peptide LL-37. J Immunol 176(4):2455–2464
Durr M, Peschel A (2002) Chemokines meet defensins: the merging concepts of chemoattractants and antimicrobial peptides in host defense. Infect Immun 70(12):6515–6517
Tjabringa GS, Aarbiou J, Ninaber DK, Drijfhout JW, Sorensen OE, Borregaard N, Rabe KF, Hiemstra PS (2003) The antimicrobial peptide LL-37 activates innate immunity at the airway epithelial surface by transactivation of the epidermal growth factor receptor. J Immunol 171(12):6690–6696
Cirioni O, Giacometti A, Ghiselli R, Bergnach C, Orlando F, Silvestri C, Mocchegiani F, Licci A, Skerlavaj B, Rocchi M, Saba V, Zanetti M, Scalise G (2006) LL-37 protects rats against lethal sepsis caused by Gram-negative bacteria. Antimicrob Agents Chemother 50(5):1672–1679. https://doi.org/10.1128/AAC.50.5.1672-1679.2006
Begde D, Bundale S, Mashitha P, Rudra J, Nashikkar N, Upadhyay A (2011) Immunomodulatory efficacy of nisin--a bacterial lantibiotic peptide. J Pept Sci 17(6):438–444. https://doi.org/10.1002/psc.1341
Brinkmann V, Zychlinsky A (2007) Beneficial suicide: why neutrophils die to make NETs. Nat Rev Microbiol 5(8):577–582. https://doi.org/10.1038/nrmicro1710
Kindrachuk J, Jenssen H, Elliott M, Nijnik A, Magrangeas-Janot L, Pasupuleti M, Thorson L, Ma S, Easton DM, Bains M, Finlay B, Breukink EJ, Georg-Sahl H, Hancock RE (2013) Manipulation of innate immunity by a bacterial secreted peptide: lantibiotic nisin Z is selectively immunomodulatory. Innate Immun 19(3):315–327. https://doi.org/10.1177/1753425912461456
Singh AP, Preet S, Rishi P (2014) Nisin/beta-lactam adjunct therapy against Salmonella enterica serovar Typhimurium: a mechanistic approach. J Antimicrob Chemother 69(7):1877–1887. https://doi.org/10.1093/jac/dku049
Silva ON, de la Fuente-Nunez C, Haney EF, Fensterseifer IC, Ribeiro SM, Porto WF, Brown P, Faria-Junior C, Rezende TM, Moreno SE, Lu TK, Hancock RE, Franco OL (2016) An anti-infective synthetic peptide with dual antimicrobial and immunomodulatory activities. Sci Rep 6. https://doi.org/10.1038/srep35465
Yeaman MR, Yount NY (2003) Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev 55(1):27–55. https://doi.org/10.1124/pr.55.1.2
Guilhelmelli F, Vilela N, Albuquerque P, Derengowski Lda S, Silva-Pereira I, Kyaw CM (2013) Antibiotic development challenges: the various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front Microbiol 4. https://doi.org/10.3389/fmicb.2013.00353
Marr AK, Gooderham WJ, Hancock RE (2006) Antibacterial peptides for therapeutic use: obstacles and realistic outlook. Curr Opin Pharmacol 6(5):468–472. https://doi.org/10.1016/j.coph.2006.04.006
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X, Yu LF, Gu D, Ren H, Chen X, Lv L, He D, Zhou H, Liang Z, Liu JH, Shen J (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16(2):161–168. https://doi.org/10.1016/S1473-3099(15)00424-7
MacNair CR, Stokes JM, Carfrae LA, Fiebig-Comyn AA, Coombes BK, Mulvey MR, Brown ED (2018) Overcoming mcr-1 mediated colistin resistance with colistin in combination with other antibiotics. Nat Commun 9(1):458. https://doi.org/10.1038/s41467-018-02875-z
Okdah L, Le Page S, Olaitan AO, Dubourg G, Hadjadj L, Rolain JM (2018) New therapy from old drugs: synergistic bactericidal activity of sulfadiazine with colistin against colistin-resistant bacteria, including plasmid-mediated colistin-resistant mcr-1 isolates. Int J Antimicrob Agents 51(5):775–783. https://doi.org/10.1016/j.ijantimicag.2018.01.027
Pag U, Sahl HG (2002) Multiple activities in lantibiotics--models for the design of novel antibiotics? Curr Pharm Des 8(9):815–833
Shin JM, Ateia I, Paulus JR, Liu H, Fenno JC, Rickard AH, Kapila YL (2015) Antimicrobial nisin acts against saliva derived multi-species biofilms without cytotoxicity to human oral cells. Front Microbiol 6. https://doi.org/10.3389/fmicb.2015.00617
Peschel A, Sahl HG (2006) The co-evolution of host cationic antimicrobial peptides and microbial resistance. Nat Rev Microbiol 4(7):529–536. https://doi.org/10.1038/nrmicro1441
Matsuzaki K (2009) Control of cell selectivity of antimicrobial peptides. Biochim Biophys Acta 1788(8):1687–1692. https://doi.org/10.1016/j.bbamem.2008.09.013
Takahashi D, Shukla SK, Prakash O, Zhang G (2010) Structural determinants of host defense peptides for antimicrobial activity and target cell selectivity. Biochimie 92(9):1236–1241. https://doi.org/10.1016/j.biochi.2010.02.023
Porto WF, Irazazabal L, Alves ESF, Ribeiro SM, Matos CO, Pires AS, Fensterseifer ICM, Miranda VJ, Haney EF, Humblot V, Torres MDT, Hancock REW, Liao LM, Ladram A, Lu TK, de la Fuente-Nunez C, Franco OL (2018) In silico optimization of a guava antimicrobial peptide enables combinatorial exploration for peptide design. Nat Commun 9(1):1490. https://doi.org/10.1038/s41467-018-03746-3
Barreto-Santamaria A, Curtidor H, Arevalo-Pinzon G, Herrera C, Suarez D, Perez WH, Patarroyo ME (2016) A new synthetic peptide having two target of antibacterial action in E. coli ML35. Front Microbiol https://doi.org/10.3389/fmicb.2016.02006
Rodriguez-Rojas A, Makarova O, Rolff J (2014) Antimicrobials, stress and mutagenesis. PLoS Pathog 10(10):e1004445. https://doi.org/10.1371/journal.ppat.1004445
Hancock RE (2015) Rethinking the antibiotic discovery paradigm. EBioMedicine 2(7):629–630. https://doi.org/10.1016/j.ebiom.2015.07.010
Prins JM, Kuijper EJ, Mevissen ML, Speelman P, van Deventer SJ (1995) Release of tumor necrosis factor alpha and interleukin 6 during antibiotic killing of Escherichia coli in whole blood: influence of antibiotic class, antibiotic concentration, and presence of septic serum. Infect Immun 63(6):2236–2242
Field D, Begley M, O'Connor PM, Daly KM, Hugenholtz F, Cotter PD, Hill C, Ross RP (2012) Bioengineered nisin a derivatives with enhanced activity against both gram positive and gram negative pathogens. PLoS One 7(10):e46884. https://doi.org/10.1371/journal.pone.0046884
Bavin EM, Beach AS, Falconer R, Friedmann R (1952) Nisin in experimental tuberculosis. Lancet 1(6699):127–129. https://doi.org/10.1016/S0140-6736(52)92429-X
Yoneyama H, Katsumata R (2006) Antibiotic resistance in bacteria and its future for novel antibiotic development. Biosci Biotechnol Biochem 70(5):1060–1075. https://doi.org/10.1271/bbb.70.1060
Smith L, Hillman J (2008) Therapeutic potential of type a (I) lantibiotics, a group of cationic peptide antibiotics. Curr Opin Microbiol 11(5):401–408. https://doi.org/10.1016/j.mib.2008.09.008
Ongey EL, Neubauer P (2016) Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production. Microb Cell Factories 15. https://doi.org/10.1186/s12934-016-0502-y
Ali MP, Yoshimatsu K, Suzuki T, Kato T, Park EY (2014) Expression and purification of cyto-insectotoxin (Cit1a) using silkworm larvae targeting for an antimicrobial therapeutic agent. Appl Microbiol Biotechnol 98(16):6973–6982. https://doi.org/10.1007/s00253-014-5728-1
Bommarius B, Jenssen H, Elliott M, Kindrachuk J, Pasupuleti M, Gieren H, Jaeger KE, Hancock RE, Kalman D (2010) Cost-effective expression and purification of antimicrobial and host defense peptides in Escherichia coli. Peptides 31(11):1957–1965. https://doi.org/10.1016/j.peptides.2010.08.008
Li Y (2011) Recombinant production of antimicrobial peptides in Escherichia coli: a review. Protein Expr Purif 80(2):260–267. https://doi.org/10.1016/j.pep.2011.08.001
Kong W, Lu T (2014) Cloning and optimization of a nisin biosynthesis pathway for bacteriocin harvest. ACS Synth Biol 3(7):439–445. https://doi.org/10.1021/sb500225r
Bell G, Gouyon PH (2003) Arming the enemy: the evolution of resistance to self-proteins. Microbiology 149(6):1367–1375. https://doi.org/10.1099/mic.0.26265-0
Fleitas O, Franco OL (2016) Induced bacterial cross-resistance toward host antimicrobial peptides: a worrying phenomenon. Front Microbiol 7. https://doi.org/10.3389/fmicb.2016.00381
Norrby R, Powel M, Aronsson B, Monnet DL, Lutsar I, Bocsan IS, Cars O, Giamarellou H, Gyssens IC (2009) ECDC/EMEA joint working group. Joint technical report: the bacterial challenge: time to react European Centre for Disease Prevention and Control https://ecdceuropaeu/en/publications-data/ecdcemea-joint-technical-report-bacterial-challenge-time-react Accessed 20 August 2017
Nwokoro E, Leach R, Ardal C, Baraldi E, Ryan K, Plahte J (2016) An assessment of the future impact of alternative technologies on antibiotics markets. J Pharm Policy Pract 9. https://doi.org/10.1186/s40545-016-0085-3
Funding
AL is grateful for financial assistance from the National Research Foundation (NRF) of South Africa (Grant number 94942). Opinions expressed and conclusions arrived at are those of the authors and are not to be attributed to the NRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lewies, A., Du Plessis, L.H. & Wentzel, J.F. Antimicrobial Peptides: the Achilles’ Heel of Antibiotic Resistance?. Probiotics & Antimicro. Prot. 11, 370–381 (2019). https://doi.org/10.1007/s12602-018-9465-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9465-0