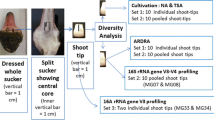
Abstract
The extracellular space in plants, termed the apoplast, has a pH and sugar content that enables bacterial growth and represents a possible niche for the establishment of endophytic bacteria. Previous studies have investigated the effects of diazotrophic bacteria inoculation in sugarcane varieties, but it has not yet been analyzed how the microbial community of apoplast fluid of sugarcane is affected. High-throughput next generation sequencing of the 16S rRNA gene was used throughout this study to determine the effect of inoculation with a diazotrophic bacteria consortium, previously isolated from sugarcane, on the native bacterial population of sugarcane variety RB867515 grown in the field. The analyses were carried out 450 days after inoculation. The results revealed the presence of 22 phyla, with predominance of Proteobacteria phylum. It was observed that the inoculated consortium changed the indigenous bacterial community structure of sugarcane apoplast fluid by decreasing diversity and evenness, interfering in the composition of rare species. Microbial community composition analysis revealed differences between treatments. The differential abundance test showed there were 43 amplicon sequence variants (ASVs) which were relatively more abundant in the inoculated treatment, with predominance of the Sphingomonas genus. The predicted functions of the most abundant ASVs revealed the presence of genera related to plant growth promotion and protection against phytopathogens. Analysis to evaluate the occurrence of inoculated strains in the recovered data was not conclusive since the ASVs taxonomically close to the inoculated bacteria were observed in low abundance. The present study is the first report to elucidate the bacterial community in sugarcane apoplast fluid using a culture-independent approach. It demonstrated that the diazotrophic bacterial consortium interferes in the natural bacterial community in sugarcane variety RB867515.





Similar content being viewed by others
References
de Oliveira Bordonal R, Carvalho JLN, Lal R et al (2018) Sustainability of sugarcane production in Brazil. A review. Agron Sustain Dev 38:13. https://doi.org/10.1007/s13593-018-0490-x
CONAB (2019) Acompanhamento de safra brasileiro—Cana-de-açúcar—Safra 2019/20. Companhia Nacional de Abastecimento 6:58
Wang J, Nayak S, Koch K, Ming R (2013) Carbon partitioning in sugarcane (Saccharum species). Front Plant Sci 4:2005–2010. https://doi.org/10.3389/fpls.2013.00201
Asis Júnior C, Adachi K, Sugimoto A et al (2003) Population of diazotrophic endophytes in stem apoplast solution of sugarcane and related grass species in Tanegashima, Japan. Microbes Environ 18:133–137. https://doi.org/10.1264/jsme2.18.133
Dawe D (2000) The potential role of biological nitrogen fixation in meeting future demand for rice and fertilizer. In: Ladha JK, Reddy PM (eds) The quest for nitrogen fixation in rice. Los Banos, Philippines
Urquiaga S, Cruz KHS, Boddey RM (1992) Contribution of nitrogen fixation to sugar cane: nitrogen-15 and nitrogen-balance estimates. Soil Sci Soc Am J 56:105. https://doi.org/10.2136/sssaj1992.03615995005600010017x
Oliveira ALM, Urquiaga S, Döbereiner J, Baldani JI (2002) The effect of inoculating endophytic N2-fixing bacteria on micropropagated sugarcane plants. Plant Soil 242:205–215. https://doi.org/10.1023/A:1016249704336
Boddey RM, Urquiaga S, Alves BJR, Reis V (2003) Endophytic nitrogen fixation in sugarcane: present knowledge and future applications. Plant Soil 252:139–149. https://doi.org/10.1023/A:1024152126541
Eskin N, Vessey K, Tian L (2014) Research progress and perspectives of nitrogen fixing bacterium, Gluconacetobacter diazotrophicus, in monocot plants. Int J Agron. https://doi.org/10.1155/2014/208383
Monteiro RA, Balsanelli E, Wassem R et al (2012) Herbaspirillum-plant interactions: microscopical, histological and molecular aspects. Plant Soil 356:175–196
de Oliveira ALM, de Canuto EL, Urquiaga S et al (2006) Yield of micropropagated sugarcane varieties in different soil types following inoculation with diazotrophic bacteria. Plant Soil 284:23–32. https://doi.org/10.1007/s11104-006-0025-0
Oliveira ALM, Stoffels M, Schmid M et al (2009) Colonization of sugarcane plantlets by mixed inoculations with diazotrophic bacteria. Eur J Soil Biol 45:106–113. https://doi.org/10.1016/j.ejsobi.2008.09.004
Reis VM, Urquiaga S, Pereira W et al (2009) Eficiência agronômica do inoculante de cana-de-açúcar aplicado em três ensaios conduzidos no Estado do Rio de Janeiro durante o primeiro ano de cultivo. Embrapa Agrobiologia. Boletim de Pesquisa e Desenvolvimento, 45:1–22
dos Santos SG, Chaves VA, da Silva RF et al (2019) Rooting and growth of pre-germinated sugarcane seedlings inoculated with diazotrophic bacteria. Appl Soil Ecol 133:12–23. https://doi.org/10.1016/j.apsoil.2018.08.015
Schultz N, Pereira W, de Albuquerque SP et al (2017) Yield of sugarcane varieties and their sugar quality grown in different soil types and inoculated with a diazotrophic bacteria consortium. Plant Prod Sci 20:366–374. https://doi.org/10.1080/1343943X.2017.1374869
Urquiaga S, Xavier RP, de Morais RF et al (2012) Evidence from field nitrogen balance and 15N natural abundance data for the contribution of biological N2 fixation to Brazilian sugarcane varieties. Plant Soil 356:5–21. https://doi.org/10.1007/s11104-011-1016-3
Hardoim PR, van Overbeek LS, Berg G et al (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320. https://doi.org/10.1128/MMBR.00050-14
Reinhold-Hurek B, Hurek T (2011) Living inside plants: bacterial endophytes. Curr Opin Plant Biol 14:435–443. https://doi.org/10.1016/j.pbi.2011.04.004
Dong Z, Canny MJ, Mccully ME et al (1994) A nitrogen-fixing endophyte of sugarcane stems (a new role for the apoplast). Plant Physiol 105:1139–1147. https://doi.org/10.1104/pp.105.4.1139
James E, Reis V, Olivares F et al (1994) Infection of sugar cane by the nitrogen-fixing bacterium Acetobacter diazotrophicus. J Exp Bot 45:757. https://doi.org/10.1093/jxb/45.6.757
James EK, Olivares FL, de Oliveira ALM et al (2001) Further observations on the interaction between sugar cane and Gluconacetobacter diazotrophicus under laboratory and greenhouse conditions. J Exp Bot 52:747–760. https://doi.org/10.1093/jexbot/52.357.747
Welbaum GE, Meinzer FC (1990) Compartmentation of solutes and water in developing sugarcane stalk tissue. Plant Physiol 93:1147–1153. https://doi.org/10.1104/pp.93.3.1147
Tejera N, Ortega E, Rodes R, Lluch C (2006) Nitrogen compounds in the apoplastic sap of sugarcane stem: some implications in the association with endophytes. J Plant Physiol 163:80–85. https://doi.org/10.1016/j.jplph.2005.03.010
Sattelmacher B (2001) The apoplast and its significance for plant mineral nutrition. New Phytol 149:167–192. https://doi.org/10.1046/j.1469-8137.2001.00034.x
Asis Júnior C, Katsuki A, Akao S (2004) N2 fixation in sugarcane and population of N2-fixing endophytes in stem apoplast solution. Philipp J Crop Sci 29:45–58
Loiret FG, Ortega E, Kleiner D et al (2004) A putative new endophytic nitrogen-fixing bacterium Pantoea sp. from sugarcane. J Appl Microbiol 97:504–511. https://doi.org/10.1111/j.1365-2672.2004.02329.x
Dos-Santos CM, de Souza DG, Balsanelli E et al (2017) A culture-independent approach to enrich endophytic bacterial cells from sugarcane stems for community characterization. Microb Ecol 74:453–465. https://doi.org/10.1007/s00248-017-0941-y
de Souza RSC, Okura VK, Armanhi JSL et al (2016) Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Nat Sci Rep. https://doi.org/10.1038/srep28774
Yeoh YK, Paungfoo-Lonhienne C, Dennis PG et al (2016) The core root microbiome of sugarcanes cultivated under varying nitrogen fertilizer application. Environ Microbiol 18:1338–1351. https://doi.org/10.1111/1462-2920.12925
Rouws LFM, Leite J, de Matos GF et al (2014) Endophytic Bradyrhizobium spp. isolates from sugarcane obtained through different culture strategies. Environ Microbiol Rep 6:354–363. https://doi.org/10.1111/1758-2229.12122
Fischer D, Pfitzner B, Schmid M et al (2012) Molecular characterisation of the diazotrophic bacterial community in uninoculated and inoculated field-grown sugarcane (Saccharum sp.). Plant Soil 356:83–99. https://doi.org/10.1007/s11104-011-0812-0
Magnani GS, Didonet CMCM, Cruz LMLM et al (2010) Diversity of endophytic bacteria in Brazilian sugarcane. Genet Mol Res 9:250–258. https://doi.org/10.4238/vol9-1gmr703
Martins DS, Reis VM, Schultz N et al (2020) Both the contribution of soil nitrogen and of biological N2 fixation to sugarcane can increase with the inoculation of diazotrophic bacteria. Plant Soil. https://doi.org/10.1007/s11104-020-04621-1
Cavalcante VA, Dobereiner J (1988) A new acid-tolerant nitrogen-fixing bacterium associated with sugarcane. Plant Soil 108:23–31. https://doi.org/10.1007/BF02370096
Baldani JI, Pot B, Kirchhof G et al (1996) Emended description of Herbaspirillum; inccusion of [Pseudomonas] rubrisubalbicans, a mild plant pathogen, as Herbaspirillum rubrisubalbicans comb. nov.; and classification of a group of clinical isolates (EF group 1) as Herbaspirillum species 3. Int J Syst Bacteriol 46:802–810. https://doi.org/10.1099/00207713-46-3-802
Baldani JI, Baldani VLD, Seldin L, Dobereiner J (1986) Characterization of Herbaspirillum seropedicae gen. nov., sp. nov., a root-associated nitrogen-fixing bacterium. Int J Syst Bacteriol 36:86–93. https://doi.org/10.1099/00207713-36-1-86
Magalhães FM, Baldani JI, Souto SM et al (1983) New acid-tolerant Azospirillum species. Anais—Academia Brasileira de Ciências 55:417–430
Lin S-Y, Hameed A, Shen F-T et al (2014) Description of Niveispirillum fermenti gen. nov., sp. nov., isolated from a fermentor in Taiwan, transfer of Azospirillum irakense (1989) as Niveispirillum irakense comb. nov., and reclassification of Azospirillum amazonense (1983) as Nitrospirillum amazonense gen. nov. Antonie Van Leeuwenhoek 105:1149–1162. https://doi.org/10.1007/s10482-014-0176-6
Reis VM, Estrada-De Los Santos P, Tenorio-Salgado S et al (2004) Burkholderia tropica sp. nov., a novel nitrogen-fixing, plant-associated bacterium. Int J Syst Evol Microbiol 54:2155–2162
Garrity GM, Oren A (2015) List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol 65:2017–2025. https://doi.org/10.1099/ijs.0.000317
Boström KH, Simu K, Hagström Å, Riemann L (2004) Optimization of DNA extraction for quantitative marine bacterioplankton community analysis. Limnol Oceanogr Methods 2:365–373. https://doi.org/10.4319/lom.2004.2.365
Caporaso JG, Lauber CL, Walters WA et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci USA 108:4516–4522. https://doi.org/10.1073/pnas.1000080107
Callahan BJ, McMurdie PJ, Rosen MJ et al (2016) DADA2: high-resolution sample inference from illumina amplicon data. Nat Methods 13:581–583. https://doi.org/10.1038/nmeth.3869
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene data base project: improved data processing and web-based tools. Nucleic Acids Res 41:gks1219. https://doi.org/10.1093/nar/gks1219
Wright ES (2016) Using DECIPHER v2.0 to analyze big biological sequence data in R. R J 8:352–359. https://doi.org/10.32614/rj-2016-025
Schliep KP (2011) phangorn: phylogenetic analysis in R. Bioinformatics 27:592–593. https://doi.org/10.1093/bioinformatics/btq706
Lozupone C, Lladser ME, Knights D et al (2011) UniFrac: an effective distance metric for microbial community comparison. ISME J 5:169–172. https://doi.org/10.1038/ismej.2010.133
McMurdie PJ, Holmes S (2013) phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 8:e61217. https://doi.org/10.1371/journal.pone.0061217
Weiss S, Xu ZZ, Peddada S et al (2017) Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome. https://doi.org/10.1186/s40168-017-0237-y
Altschul SF, Gish W, Miller W et al (1990) Basic local alignment search tool. J Mol Biol 215:403–410. https://doi.org/10.1016/S0022-2836(05)80360-2
Larkin MA, Blackshields G, Brown NP et al (2007) Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948
He Z, Zhang H, Gao S et al (2016) Evolview v2: an online visualization and management tool for customized and annotated phylogenetic trees. Nucleic Acids Res 44:W236–W241
Dixon P (2003) VEGAN, a package of R functions for community ecology. J Veg Sci 14:927–930. https://doi.org/10.1111/j.1654-1103.2003.tb02228.x
Chao A (1984) Nonparametric estimation of the number of classes in a population. Scand J Stat 11(4):265–270
Shannon CE (1949) The mathematical theory of information. MD Comput: Comput Med Pract 14:306–317
Simpson EH (1949) Measurement of diversity. Nature 163:688–688. https://doi.org/10.1038/163688a0
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc: Ser B (Methodol) 57:289–300
Aßhauer KP, Wemheuer B, Daniel R, Meinicke P (2015) Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31:2882–2884. https://doi.org/10.1093/bioinformatics/btv287
Reis VM, Olivares FL, Döbereiner J (1994) Improved methodology for isolation of Acetobacter diazotrophicus and confirmation of its endophytic habitat. World J Microbiol Biotechnol 10:401–405. https://doi.org/10.1007/BF00144460
Dong Z, McCully ME, Canny MJ (1997) Does Acetobacter diazotrophicus live and move in the xylem of sugarcane stems? Anatomical and physiological data. Ann Bot 80:147–158. https://doi.org/10.1006/anbo.1997.0426
Velázquez E, Rojas M, Lorite MJM et al (2008) Genetic diversity of endophytic bacteria which could be find in the apoplastic sap of the medullary parenchym of the stem of healthy sugarcane plants. J Basic Microbiol 48:118–124. https://doi.org/10.1002/jobm.200700161
Andreote FD, Da RUN, Araújo WL et al (2010) Effect of bacterial inoculation, plant genotype and developmental stage on root-associated and endophytic bacterial communities in potato (Solanum tuberosum). Antonie van Leeuwenhoek, Int J Gen Mol Microbiol 97:389–399. https://doi.org/10.1007/s10482-010-9421-9
Gadhave KR, Devlin PF, Ebertz A et al (2018) Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb Ecol 76:741–750. https://doi.org/10.1007/s00248-018-1160-x
Armanhi JSL, de Souza RSC, Damasceno NDB et al (2018) A community-based culture collection for targeting novel plant growth-promoting bacteria from the sugarcane microbiome. Front Plant Sci 8:1–17. https://doi.org/10.3389/fpls.2017.02191
Kim BR, Shin J, Guevarra RB et al (2017) Deciphering diversity indices for a better understanding of microbial communities. J Microbiol Biotechnol 27:2089–2093. https://doi.org/10.4014/jmb.1709.09027
Baldani JI, Baldani VLD (2005) History on the biological nitrogen fixation research in graminaceous plants: special emphasis on the Brazilian experience. Anais da Academia Brasileira de Ciencias 77:549–579. https://doi.org/10.1590/S0001-37652005000300014
Baldani JI, Reis VM, Videira SS et al (2014) The art of isolating nitrogen-fixing bacteria from non-leguminous plants using N-free semi-solid media: a practical guide for microbiologists. Plant Soil 384:413–431. https://doi.org/10.1007/s11104-014-2186-6
Muñoz-Rojas J, Caballero-Mellado J (2003) Population dynamics of Gluconacetobacter diazotrophicus in sugarcane cultivars and its effect on plant growth. Microb Ecol 46:454–464. https://doi.org/10.1007/s00248-003-0110-3
Trabelsi D, Mhamdi R (2013) Microbial inoculants and their impact on soil microbial communities: a review. Biomed Res Int. https://doi.org/10.1155/2013/863240
Oliveira ALM, Canuto EL, Silva EE et al (2004) Survival of endophytic diazotrophic bacteria in soil under different moisture levels. Braz J Microbiol 35:295–299
Júnior FBR, Silva LG, Reis VM, Döbereiner J (2000) Ocorrência de bactérias diazotróficas em diferentes genótipos de cana-de-açúcar. Pesq Agrop Brasileira 35:985–994
Gomes AA, Reis VM, Baldani VLD, Goi SR (2005) Relação entre distribuição de nitrogênio e colonização por bactérias diazotróficas em cana-de-açúcar. Pesq Agrop Brasileira 40:1105–1113
da Silva PRA, Vidal MS, Soares CD et al (2018) Sugarcane apoplast fluid modulates the global transcriptional profile of the diazotrophic bacteria Paraburkholderia tropica strain Ppe8. PLoS ONE 13:e0207863. https://doi.org/10.1371/journal.pone.0207863
Terra LA, de Soares CP, Meneses CHSG et al (2019) Transcriptome and proteome profiles of the diazotroph Nitrospirillum amazonense strain CBAmC in response to the sugarcane apoplast fluid. Plant Soil. https://doi.org/10.1007/s11104-019-04201-y
Pessoa DDV, Dos-Santos CM, Vidal MS, Baldani JI, Tadra-Sfeir MZ, de Souza EM, Simoes-Araujo JL (2021) Herbaspirillum seropedicae strain HRC54 expression profile in response to sugarcane apoplastic fluid. Biotech 11:292. https://doi.org/10.1007/s13205-021-02848-y
Acknowledgements
We thank Segundo Urquiaga (Embrapa Agrobiologia) and Doãn S. Martins (UFRRJ) for providing the stem sugarcane samples from the field experiment. We are grateful to the students at Embrapa Agrobiologia in the Laboratory of Genetics and Biochemistry (LGB) for their work with apoplast processing and Michelle Z. T. Sfeir and Valter A. de Baura (Universidade Federal do Paraná) for help with Illumina sequencing of the samples.
Funding
This work was partially financed by the projects CNPq/INCT-FBN (573828/2008-3), FAPERJ–CNE (E-26/203.011/2016), and Embrapa MP2 (02.16.05.017.00.00). CMS and NVSR were supported by a fellowship from FAPERJ (E-26/200.952/2018) and (E-26/202.015/2019), respectively; JIB (306011/2017-4) received a fellowship from CNPq.
Author information
Authors and Affiliations
Contributions
MSV, JIB and SS designed and directed the project. NVSR carried out the experimental analysis. CMD-S performed and interpreted the results of the bioinformatics and statistical analysis. All authors discussed the results and commented on the manuscript. CMD-S wrote the manuscript with support from SS, JIB and MSV. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
284_2021_2571_MOESM2_ESM.tif
Supplementary file2 (TIF 2027 kb) Molecular profiles of 16S rRNA gene amplicons obtained using primers 799F and 1492R. Bands with ~1,000 bp and ~700 bp are related to plant (mitochondrial) and bacterial amplicons, respectively. M, 1 kb Plus DNA ladder (Invitrogen); N, PCR negative control; P, PCR positive control (using DNA from a bacterial control)
284_2021_2571_MOESM3_ESM.xlsx
Supplementary file3 (XLSX 273 kb) List of ASVs detected in the present study, respectively number of reads and relatives abundances for each sample, taxonomical classification and its treatment detection
284_2021_2571_MOESM4_ESM.xlsx
Supplementary file4 (XLSX 49 kb) The ASVs differentially abundant observed by DAT. Log2FoldChange values < 0.00 represents low abundant ASVs in the inoculated treatment and > 0.00 more abundant ASVs
284_2021_2571_MOESM5_ESM.xlsx
Supplementary file5 (XLSX 259 kb) Predicted functions capabilities of the more abundant ASVs of the inoculated treatment and the mined genes related to plant growth promotion
Rights and permissions
About this article
Cite this article
Dos-Santos, C.M., Ribeiro, N.V.S., Schwab, S. et al. The Effect of Inoculation of a Diazotrophic Bacterial Consortium on the Indigenous Bacterial Community Structure of Sugarcane Apoplast Fluid. Curr Microbiol 78, 3079–3091 (2021). https://doi.org/10.1007/s00284-021-02571-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-021-02571-0