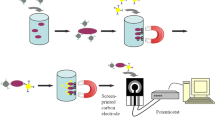
Abstract
The need for alternative approaches for identifying pathogens has led researchers to focus on nanobiotechnology. In this study, zinc oxide nanoparticles (ZnO NPs) and multi-wall carbon nanotubes (MWCNTs) were used as marker molecules. After measuring the best concentration of these nanomaterials to inhibit the lactase activity of the beta-galactosidase enzymes by binding to them, different concentrations of Escherichia coli were added to the medium and their detection ability was finally compared with each other. Due to small size and high reactivity, these compounds are able to detect very low amount of bacteria in the ambient. In fact, the bacteria are attached to the nanoparticles and detach them from the enzyme and lead to substrate decomposition by the enzyme. MWCNTs exhibited better performance than ZnO NPs in detection of bacteria at very low concentration of 101 CFU/ml in 15 min. As a result, they are very appropriate to be utilized especially in the food industry.





Similar content being viewed by others
References
Jannat Y, Mohammed Raju A, Byoung-Kwan C (2016) Biosensors and their applications in food safety: a review. J Biosyst Eng 41:240–254. https://doi.org/10.5307/JBE.2016.41.3.240
Mehrotra P (2016) Biosensors and their applications: a review. J Oral Biol Craniofac Res 6:153–159. https://doi.org/10.1016/j.jobcr.2015.12.002
Ahari H (2014) The Staphylococcus aureus exotoxin recognition using a Sensor designed by nanosilica and SEA genotyping by multiplex PCR. Appl Food Biotechnol 1:37–44. https://doi.org/10.22037/afb.v1i1.7126
Chai Y, Horikawa S, Simonian A, Dyer D, Chin BA (2013) Wireless magnetoelastic biosensors for the detection of Salmonella on fresh produce. Proc Int Conf Sens Technol ICST 204:174–177
Ahmed A, Rushworth JV, Hirst NA, Millner PA (2014) Biosensors for whole-cell bacterial detection. Clin Microbiol Rev 27(3):631–646. https://doi.org/10.1128/CMR.00120-13
Choi Y et al (2018) Rapid detection of Escherichia coli in fresh foods using a combination of enrichment and PCR analysis. Korean J Food Sci Anim Resour 38:829–834. https://doi.org/10.5851/kosfa.2018.e19
Yamada K, Kim CT, Kim JH, Chung JH, Lee HG, Jun S (2014) Single walled carbon nanotube-based junction biosensor for detection of Escherichia coli. PLoS ONE. https://doi.org/10.1371/journal.pone.0105767
Ali MA, Eldin TAS, Moghazy GME, Tork IM, Omara II (2014) Original research article detection of E. coli O157: H7 in feed samples using gold nanoparticles sensor. Int J Curr Microbiol Appl Sci 3(6):697–708
Clark LC, Lyons C (1962) Electrode systems for continuous monitoring in cardiovascular surgery. Ann N Y Acad Sci 102:29–45. https://doi.org/10.1111/j.1749-6632.1962.tb13623.x
Nguyen HH, Lee SH, Lee UJ, Fermin CD, Kim M (2019) Immobilized enzymes in biosensor applications. Materials. https://doi.org/10.3390/ma12010121
Lerner MB, Goldsmith BR, McMillon R, Dailey J, Pillai S, Singh SR, Johnson ATC (2011) A carbon nanotube immunosensor for Salmonella. AIP Adv 1(4):042127. https://doi.org/10.1063/1.3658573
Zhao Z, Lei W, Zhang X, Wang B, Jiang H (2010) ZnO-based amperometric enzyme biosensors. Sensors 10:1216–1231. https://doi.org/10.3390/s100201216
Malik P, Katyal V, Malik V, Asatkar A, Inwati G, Mukherjee TK (2013) Nanobiosensors: concepts and variations. ISRN Nanomater. https://doi.org/10.1155/2013/327435
Ansari SA, Husain Q (2011) Bioaffinity based immobilization of almond (Amygdalus communis) β-galactosidase on con A-layered calcium alginate-cellulose beads: its application in lactose hydrolysis in batch and continuous mode. Iran J Biotechnol 9:290–301
Paul B, Panigrahi AK, Singh V, Singh SG (2017) A multi-walled carbon nanotube-zinc oxide nanofiber based flexible chemiresistive biosensor for malaria biomarker detection. Analyst 142:2128–2135
Lin SY, Chang SJ, Hsueh TJ (2014) ZnO nanowires modified with Au nanoparticles for nonenzymatic amperometric sensing of glucose. Appl Phys Lett 104:193704. https://doi.org/10.1063/1.4875028
Samadi M, Shivaee HA, Zanetti M, Pourjavadi A, Moshfegh A (2012) Visible light photocatalytic activity of novel MWCNT-doped ZnO electrospun nanofibers. J Mol Catal A Chem 359:42–48. https://doi.org/10.1016/j.molcata.2012.03.019
Meraat R, Ziabari AA, Issazadeh K, Shadan N, Jalali KM (2016) Synthesis and characterization of the antibacterial activity of zinc oxide nanoparticles against Salmonella typhi. Acta Metall Sin (English Lett) 29:601–608. https://doi.org/10.1007/s40195-016-0439-5
Cullity BD (1978) Elements of X-ray diffraction, 2nd edn. Addison-Wesley, Phillippines
Juan JC, Jiang Y, Meng X, Cao W, Yarmo MA, Zhang J (2007) Supported zirconium sulfate on carbon nanotubes as water-tolerant solid acid catalyst. Mater Res Bull 42:1278–1285. https://doi.org/10.1016/j.materresbull.2006.10.017
Zhang X, Fu J, Zhang Y, Lei L (2008) A nitrogen functionalized carbon nanotube cathode for highly efficient electrocatalytic generation of H2O2 in Electro-Fenton system. Sep Purif Technol 64:116–123. https://doi.org/10.1016/j.seppur.2008.07.020
Alias SS, Ismail AB, Mohamad AA (2010) Effect of pH on ZnO nanoparticle properties synthesized by sol-gel centrifugation. J Alloys Compd 499:231–237. https://doi.org/10.1016/j.jallcom.2010.03.174
Garg P, Singh BP, Kumar G, Gupta T, Pandey I, Seth RK, Tandon RP, Mathur RB (2011) Effect of dispersion conditions on the mechanical properties of multi-walled carbon nanotubes based epoxy resin composites. J Polym Res 18:1397–1407. https://doi.org/10.1007/s10965-010-9544-8
Hu CY, Xu YJ, Duo SW, Zhang RF, Li MS (2009) Non-covalent functionalization of carbon nanotubes with surfactants and polymers. J Chin Chem Soc 56:234–239. https://doi.org/10.1002/jccs.200900033
Kavinkumar T, Manivannan S (2016) Synthesis, characterization and gas sensing properties of graphene oxide-multiwalled carbon nanotube composite. J Mater Sci Technol 32:626–632. https://doi.org/10.1016/j.jmst.2016.03.017
Mlichová Z, Rosenberg M (2006) Current trends of beta-galactosidase application in food technology. J Food Nutr Res 45:47–54
Luo Z, Zhu M, Guo M, Lian Z, Tong W, Wang J, Zhang B, Wei W (2017) Ultrasonic-assisted dispersion of ZnO nanoparticles and its inhibition activity to Trichoderma viride. J Nanosci Nanotechnol 18:2352–2360. https://doi.org/10.1166/jnn.2018.14397
Ong CB, Annuar MSM (2018) Immobilization of cross-linked tannase enzyme on multiwalled carbon nanotubes and its catalytic behavior. Prep Biochem Biotechnol 48:181–187. https://doi.org/10.1080/10826068.2018.1425707
Wibowo KM, Ahmad SA, Sari Y, Saim H, Nurliyana MR, Mansor Z, Sahdan MZ, Muslihati A (2018) The detection method of Escherichia coli in water resources: a review. J Phys Conf Ser 995:012065. https://doi.org/10.1088/1742-6596/995/1/012065
Li Z, Ma J, Ruan J, Zhuang X (2019) Using positively charged magnetic nanoparticles to capture bacteria at ultralow concentration. Nanoscale Res Lett 14:195. https://doi.org/10.1186/s11671-019-3005-z
Hwang JY, Eltohamy M, Kim HW, Shin US (2012) Self-assembly of positively charged carbon nanotubes with oppositely charged metallic surface. Appl Surf Sci 258:6455–6459. https://doi.org/10.1016/j.apsusc.2012.03.060
Andrade CAS, Nascimento JM, Oliveira IS, De Oliveira CVJ, De Melo CP, Franco OL, Oliveira MDL (2015) Nanostructured sensor based on carbon nanotubes and clavanin A for bacterial detection. Colloids Surf B Biointerfaces 135:833–839. https://doi.org/10.1016/j.colsurfb.2015.03.037
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Meraat, R., Issazadeh, K., Abdolahzadeh Ziabari, A. et al. Rapid Detection of Escherichia coli by β-Galactosidase Biosensor Based on ZnO NPs and MWCNTs: A Comparative Study. Curr Microbiol 77, 2633–2641 (2020). https://doi.org/10.1007/s00284-020-02040-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-020-02040-0