Abstract
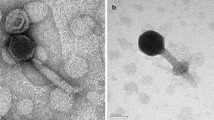
A novel cyanophage, S-B64, which can infect marine Synechococcus WH8102, was isolated from the coastal waters of the Yellow Sea using the liquid serial dilution method. Morphological study by transmission electron microscopy revealed that the cyanophage belongs to Podovirus. It’s genome, which was completely sequenced, contains a 151,867 bp DNA molecule with a G+C content of 41.78% and 186 potential open reading frames. The functions of the genes include cyanophage structure, cyanophage packaging, DNA replication and regulation. After primary characterization, it was found that the latent period is about 3 h, and it lysed after 8 h, the burst size is about 23 virions per cell. This information will provide an important benchmark for further research on the interaction between cyanophages and their hosts.




Similar content being viewed by others
References
Waterbury J et al (1986) Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Science 214:71–120
Roitman S et al (2018) Cyanophage-encoded lipid desaturases: oceanic distribution, diversity and function. ISME J 12(2):343–355
Murata K et al (2017) Visualizing adsorption of cyanophage P-SSP7 onto marine Prochlorococcus. Sci Rep 7:44176
Lu J et al (2001) Distribution, isolation, host specificity, and diversity of cyanophages infecting marine Synechococcus spp. in river estuaries. Appl Environ Microbiol 67(7):3285–3290
Suttle CA (2000) Cyanophages and their role in the ecology of cyanobacteria. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria. Springer, Dordrecht, pp 563–589
Wilson WH, Joint IR, Carr NG et al (1993) Isolation and molecular characterization of five marine cyanophages propagated on Synechococcus sp. strain WH7803. Appl Environ Microbiol 59(11):3736–3743
Labrie SJ et al (2013) Genomes of marine cyanopodoviruses reveal multiple origins of diversity. Environ Microbiol 15(5):1356–1376
Sullivan MB et al (2003) Cyanophages infecting the oceanic cyanobacterium Prochlorococcus. Nature 424(6952):1047–1051
Adriaenssens EM et al (2014) Using signature genes as tools to assess environmental viral ecology and diversity. Appl Environ Microbiol 80(15):4470–4480
Lindell D et al (2007) Genome-wide expression dynamics of a marine virus and host reveal features of co-evolution. Nature 449(7158):83–86
Zhang Y et al (2013) The first isolation of a cyanophage-synechococcus system from the east China sea. Virol Sin 28(5):260–265
Wilhelm Steven W et al (2006) Marine and freshwater cyanophages in a laurentian great lake: evidence from infectivity assays and molecular analyses of g20 genes. Appl Environ Microbiol 72(7):4957–4963
Deveau H et al (2006) Biodiversity and classification of lactococcal phages. Appl Environ Microbiol 72(6):4338–4346
Goderdzishvili et al (2013) Isolation and characterisation of lytic bacteriophages of Klebsiella pneumoniae and Klebsiella oxytoca. Curr Microbiol 66(3):251–258
Waterbury JB et al (1993) Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol 59(10):3393–3399
Xu Y et al (2016) Characterization, genome sequence, and analysis of Escherichia phage CICC 80001, a bacteriophage infecting an efficient l-aspartic acid producing Escherichia coli. Food Environ Virol 8(1):1–9
Suttle CA et al (1993) Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphology, cross-infectivity and growth characteristics. Mar Ecol Prog 92(1-2):99–109
Mann NH et al (2003) Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol Rev 27(1):17–34
Lindell D et al (2005) Photosynthesis genes in marine viruses yield proteins during host infection. Nature 438(7064):86
Sabehi G et al (2012) A novel lineage of myoviruses infecting cyanobacteria is widespread in the oceans. Proc Natl Acad Sci USA 109(6):2037–2042
Acknowledgments
This study was support by the fund from the National Natural Science Foundation of China (No. 31500339, 41676178 and 41076088), and the Marine Scientific and Technological Innovation Project Financially Supported by Pilot National Laboratory for Marine Science and Technology (Qingdao) (2018SDKJ0406-6, 2016ASKJ14), at the same time, thanks are due to the Fundamental Research Funds for the Central University of Ocean University of China (Grant nos. 201812002, 201762017, 201562018).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
You, S., Wang, M., Jiang, Y. et al. The Genome Sequence of a Novel Cyanophage S-B64 from the Yellow Sea, China. Curr Microbiol 76, 681–686 (2019). https://doi.org/10.1007/s00284-019-01680-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-019-01680-1