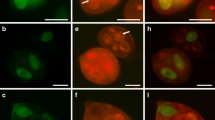
Abstract
The viability of conidia of Esteya vermicola, a potentially important biocontrol agent against the pinewood nematode Bursaphelenchus xylophilus, is usually determined by cultivation for 18–48 h in culture medium. As an alternative to this labor-intensive method, we have developed a rapid, simple, and low-cost staining method for assessing E vermicola conidia survival rates. A mixture of neutral red and methylene blue was found to be the most optimal among several stains that also included safranin O and Janus green B. This mixture stained nonviable conidia blue, in contrast to viable conidia, which were stained red in the cytoplasm and blue in the cell wall. This method may be particularly useful for traditional research laboratories, as it provides rapid results using common, relatively inexpensive laboratory equipment.


Similar content being viewed by others
References
Àlvarez G, González M, Isabal S, Blanc V, León R (2013) Method to quantify live and dead cells in multi-species oral biofilm by real-time PCR with propidium monoazide. AMB Express 3(1):1. doi:10.1186/2191-0855-3-1
Berliner MD, Reca ME (1967) Vital staining of Histoplasma capsulatum with Janus Green B. Med Mycol 5:26–29
Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single singlecell analyses. Microbiol Rev 60:641–696
Davis R, Deering A, Burgula Y, Mauer LJ, Reuhs BL (2012) Differentiation of live, dead and treated cells of Escherichia coli O157: H7 using FT-IR spectroscopy. J Appl Microbiol 112:743–751
Deere D, Shen J, Vesey G, Bell P, Bissinger P, Veal D (1998) Flow cytometry and cell sorting for yeast viability assessment and cell selection. Yeast 14:147–160
Ehara M, Noguchi T, Ueda K (1996) Uptake of neutral red by the vacuoles of a green alga, Micrasterias pinnatifida. Plant Cell Physiol 37:734–741
Guilliermond P, Gautheret R (1938) Culture de vegelaux en milieu additiones de colorants. Degre de toxicite des colorants. C R Acad Sci 206:1601 1504
Knaysi G (1935) A microscopic method of distinguishing dead from living bacterial cells. J Bacteriol 30:193
Kramer M, Obermajer N, Matijašić BB, Rogelj I, Kmetec V (2009) Quantification of live and dead probiotic bacteria in lyophilised product by real-time PCR and by flow cytometry. Appl Microbiol Biotechnol 84:1137–1147
Liou JY, Shih JY, Tzean SS (1999) Esteya, a new nematophagous genus from Taiwan, attacking the pinewood nematode (Bursaphelenchus xylophilus). Mycol Res 103:242–248
Mills DR (1941) Differential staining of living and dead yeast cells. J Food Sci 6:361–371
Milner RJ, Huppatz RJ, Swaris SC (1991) A new method for assessment of germination of Metarhizium conidia. J Invertebr Pathol 57:121–123
Norn MS (1967) Methylene blue (methyl thionine) vital staining of the cornea and conjunctiva. Acta Ophthalmol 45:347–358
Rpetto G, del Peso A, Zurita JL (2008) Neural red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 3:1125–1132
Saint-Ruf C, Cordier C, Mégret J, Matic I (2010) Reliable detection of dead microbial cells by using fluorescent hydrazides. Appl Environ Microbiol 76:1674–1678
Tzean SS, Liou JY, Shih JY (2001) Nematophagous fungus Esteya vermicola. US006168947B1[P]
Vairo ML (1961) Methylene blue solutions for staining dead yeast cells. Biotech Histochem 36:329–330
Wang CY, Fang ZM, Sun BS, Gu LJ et al (2008) High infectivity of an endoparasitic fungus strain, Esteya vermicola, against nematodes. J Microbiol 46:380–389
Wang CY, Fang ZM, Wang Z, Gu LJ et al (2009) High infection activities of two Esteya vermicola, isolates against pinewood nematode. Afr J Microbiol Res 3:581–584
Wang CY, Wang Z, Fang ZM, Zhang DL, Gu LJ, Liu L, Sung CK (2010) Attraction of pinewood nematode to endoparasitic nematophagous fungus Esteya vermicola. Curr Microbiol 60(5):387–392
Wang CY, Fang ZM, Wang Z, Zhang DL et al (2011) Biological control of the pinewood nematode Bursaphelenchus Xylophilus by application of the endoparasitic fungi Esteya vermicola. Biol Control 56:91–100
Winckler J (1974) Vital staining of lysosomes and other cell organelles of the rat with neutral Red. Prog Histochem Cytochem 6:1–89
Yamamoto DT, Uchida JY (1982) Rapid nuclear staining of Rhizoctonia solani and related fungi with acridine orange and with safranin O. Mycologia 74:145–149
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Thang, N., Li, Z. et al. A Staining Method for Assessing the Viability of Esteya vermicola Conidia. Curr Microbiol 69, 53–55 (2014). https://doi.org/10.1007/s00284-014-0553-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-014-0553-4