Abstract
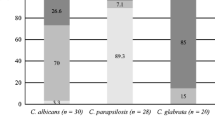
Candidosis has been attributed to C. albicans; however, infections caused by non-Candida albicans Candida (NCAC) species are increasingly being recognised. The ability of Candida to grow as a biofilm is an important feature that promotes both infection and persistence in the host. The biofilms’ activity is significant since high activity might be associated with enhanced expression of putative virulence factors, whilst in contrast low activity has previously been suggested as a mechanism for resistance of biofilm cells to antimicrobials. The aim of this study was to determine the metabolic activity of in vitro biofilms formed by different clinical isolates of NCAC species. The in situ total metabolic activity of C. parapsilosis, C. tropicalis and C. glabrata biofilms was determined using 2,3-(2-methoxy-4-nitro-5-sulphophenyl)-5-[(phenylamino) carbonyl]-2H-tetrazolium hydroxide (XTT) reduction assay, and the number of cultivable cells was also established by CFU (colony forming unit) counts. The biofilm structure was assessed by scanning electron microscopy (SEM). Results showed that total biofilm metabolic activity was species and strain dependent. C. glabrata exhibited the lowest biofilm metabolic activity despite having the highest number of biofilm cultivable cells. Similarly, the metabolic activity of resuspended C. glabrata biofilm and planktonic cells was lower than that of the other species. This study demonstrates the existence of intrinsic activity differences amongst NCAC species, which could have important implications in terms of species relative virulence. Furthermore, the absence of an obvious correlation, between cultivable cells number and total biofilm activity, raises the question about which parameter is the most appropriate for the in vitro assessment of biofilms and their potential clinical significance.



Similar content being viewed by others
References
Al-Fattani MA, Douglas LJ (2006) Biofilm matrix of Candida albicans and Candida tropicalis: chemical composition and role in drug resistance. J Med Microbiol 55:999–1008
Baillie GS, Douglas LJ (1999) Role of dimorphism in the development of Candida albicans biofilm. J Med Microbiol 48:671–679
Baillie GS, Douglas LJ (2000) Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother 46:397–403
Bassetti M, Righi E, Costa A et al (2006) Epidemiological trends in nosocomial candidemia in intensive care. BMC Infect Dis 6:21
Chandra J, Kuhn DM, Mukherjee PK et al (2001) Biofilm formation by the fungal pathogen Candida albicans: development, architecture and drug resistance. J Bacteriol 183:5385–5394
Colombo AL, Perfect J, DiNubile M et al (2003) Global distribution and outcomes for Candida species causing invasive candidiasis: results from an international randomized double-blind study of caspofungin versus amphotericin B for the treatment of invasive candidiasis. Eur J Microbiol Infect Dis 22:470–474
Costerton JW, Lewandowski Z, Caldwell DE et al (1995) Microbial biofilms. Annu Rev Microbial 49:711–745
Douglas LJ (2003) Candida biofilms and their role in infection. Trends Microbiol 11:30–36
Fidel PL, Vazquez JA, Sobel JD (1999) Candida glabrata: review of epidemiology, pathogenesis, and clinical disease with comparison to C. albicans. Clin Microbiol Rev 12:80–96
Hargety JA, Ortiz J, Reich D et al (2003) Fungal infections in solid organ transplant patients. Surg Infect (Larchmt) 4:263–271
Hasan F, Xess I, Wang X et al (2009) Biofilm formation in clinical Candida isolates and its association with virulence. Microbes Infect 11:753–761
Hawser SP, Douglas LJ (1994) Biofilm formation by Candida species on the surface of catheter materials in vitro. Infect Immun 62:915–921
Hawser SP (1996) Comparisons of the susceptibility of planktonic and adherent Candida albicans to antifungal agents: a modified XTT tetrazolium assay using synchronized C. albicans cells. J Med Vet Mycol 34:149–152
Hawser SP (1998) Production of extracellular matrix by Candida albicans biofilms. J Med Microbiol 47:253–256
Henriques M, Azeredo J, Oliveira R (2006) Candida albicans and Candida dubliniensis: comparison of biofilm formation in terms of biomass and activity. Brit J Biomed Sci 63:5–11
Jin Y, Yip KH, Samaranayake YH et al (2003) Biofilm-forming ability of Candida albicans is unlikely to contribute to high levels of oral yeast carriage in cases of human immunodeficiency virus infection. J Clin Microbiol 41:2961–2967
Kiehn TE, Edwards FF, Armstrong D (1980) The prevalence of yeasts in clinical specimens from cancer patients. Am J Clin Pathol 73:518–521
Kuhn DM, Chandra J, Mukherjee PK et al (2002) Comparison of biofilms formed by Candida albicans and Candida parapsilosis on bioprosthetic surfaces. Infect Immunol 70:878–888
Kuhn DM, Balkis M, Chandra J et al (2003) Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J Clin Microbiol 41:506–508
Kwon-Chung KJ, Bennett JE (1992) Dermatophytoses. In: Medical mycology, 1st edn. Lea & Febiger, Pennsylvania, pp. 105–161
Mah TFC, Toole GAO (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9:34–39
Mukherjee PK, Chandra J, Kuhn DM et al (2003) Mechanism of fluconazole resistance in Candida albicans biofilms: phase-specific role of efflux pumps and membrane sterols. Infect Immun 71:4333–4340
Odds FC (1994) Pathogenesis of Candida infections. J Am Acad Dermatol 31:2–5
Odds FC (1998) Candida and candidosis, 2nd edn. Bailliere Tindall, London
Ramage G, Saville PS, Thomas PD et al (2005) Candida biofilms: an update. Eukaryot Cell 4:633–638
Samaranayake LP (1990) Host factors and oral candidosis. In: Oral candidosis. Butterworth &Co (Publishers), London, pp 66–103
Samaranayake LP, Fidel PL, Naglik JR et al (2002) Fungal infections associated with HIV infection. Oral Dis 8:151–160
Shin JH, Kee SJ, Shin MG et al (2002) Biofilm production by isolates of Candida species recovered from nonneutropenic patients: comparison of bloodstream isolates with isolates from other sources. J Clin Microbiol 40:1244–1248
Williams DW, Wilson MJ, Lewis MAO et al (1995) Identification of Candida species by PCR and restriction fragment length polymorphism analysis of intergenic spacer regions of ribosomal DNA. J Clin Microbiol 33:2476–2479
Zaw MT, Samaranayake YH, Samaranayake LP (2007) In vitro biofilm formation of Candida albicans and non-albicans Candida species under dynamic and anaerobic conditions. Arch Oral Biol 52:761–767
Acknowledgements
The authors acknowledge ‘Fundação para a Ciência e Tecnologia (FCT), Portugal, for supporting Sonia Silva’s study through grant SFRH/BD/28341/2006 and project PDTC/BIO/61112/2004. The authors are also grateful to Hospital de S Marcos, Braga for providing the clinical isolates.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva, S., Henriques, M., Oliveira, R. et al. In Vitro Biofilm Activity of Non-Candida albicans Candida Species. Curr Microbiol 61, 534–540 (2010). https://doi.org/10.1007/s00284-010-9649-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9649-7