Abstract
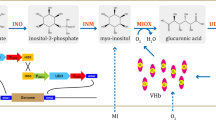
Dihydroxyacetone (DHA) is an important ketose sugar, which is extensively used in the cosmetic, chemical, and pharmaceutical industries. DHA has been industrially produced by Gluconobacter oxydans with a high demand of oxygen. To improve the production of DHA, the gene vgb encoding Vitreoscilla hemoglobin (VHb) was successfully introduced into G. oxydans, where it was stably maintained, and expressed at about 76.0 nmol/g dry cell weight. Results indicated that the constitutively expressed VHb improved cell growth and DHA production in G. oxydans under different aeration conditions. Especially at low aeration rates, the VHb-expressing strain (VHb+) displayed 23.13% more biomass and 37.36% more DHA production than those of VHb-free strain (VHb−) after 32 h fermentation in bioreactors. In addition, oxygen uptake rate (OUR) was also increased in VHb+ strain relative to the control strain during fermentation processes.




Similar content being viewed by others
References
Adlercreutz P, Mattiasson B (1982) Oxygen supply to immobilized cells: oxygen supply by hemoglobin or emulsions of perfluorochemicals. Eur J Appl Microbiol Biotechnol 16:165–170
Adlercreutz P, Mattiasson B (1984) Oxygen supply to immobilized cells: use of p-benzoquinone as an oxygen substitute. Appl Microbiol Biotechnol 20:296–302
Bhave SL, Chattoo BB (2003) Expression of Vitreoscilla hemoglobin improves growth and levels of extracellular enzyme in Yarrowia lipolytica. Biotechnol Bioeng 84:658–666
Claret C, Salmon JM, Romieu C, Bories A (1994) Physiology of Gluconobacter oxydans during dihydroxyacetone production from glycerol. Appl Microbiol Biotechnol 41:359–365
Dikshit KL, Webster DA (1988) Cloning, characterization and expression of the bacteria globin gene from Vitreoscilla in Escherichia coli. Gene 70:377–386
Gupta A, Singh VK, Qazi GN, Kumar A (2001) Gluconobacter oxydans: its biotechnological applications. J Mol Microbiol Biotechnol 3:445–456
Hekmat D, Bauer R, Fricke J (2003) Optimization of the microbial synthesis of dihydroxyacetone from glycerol with Gluconobacter oxydans. Bioprocess Biosyst Eng 26:109–116
Hoist O, Lundbäck H, Mateiasson B (1985) Hydrogen peroxide as an oxygen source for immobilized Gluconobacter oxydans converting glycerol to dihydroxyacetone. Appl Microbiol Biotechnol 22:383–388
Kallio PT, Kim DJ, Tsai PS, Baily JE (1994) Intracellular expression of Vitreoscilla hemoglobin alters Escherichia coli energy metabolism under oxygen-limited conditions. Eur J Biochem 219:201–208
Khosla C, Bailey JE (1989) Characterization of the oxygen-dependent promoter of the Vitreoscilla hemoglobin gene in Escherichia coli. J Bacteriol 171:5995–6004
Khosla C, Curtis JE, Bydalek P, Swartz JR, Bailey JE (1990) Expression of recombinant proteins in Escherichia coli using an oxygen-responsive promoter. Nat Biotechnol 8:554–558
Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM II, Peterson KM (1995) Four new derivatives of the broad-host-range cloning vector pBBR1MCS carrying different antibiotic resistance cassettes. Gene 166:175–176
Leung R, Poncelet D, Neufeld RJ (1997) Enhancement of oxygen transfer rate using microencapsulated silicone oils as oxygen carriers. J Chem Technol Biotechnol 68:37–46
Liu CY, Webster DA (1974) Spectral characteristics and interconversions of the reduced, oxidized, oxygenated forms of purified cytochrome o. J Biol Chem 249:4261–4266
Makhotkina TA, Pomortseva NV, Lomova IE, Nikolaev PI (1981) Glycerol transformations into dihydroxyacetone by polyacrylamide gel immobilized cells of Gluconobacter oxydans. Prikl Biokhim Mikrobiol 17:102–106
Merfort M, Herrmann U, Bringer-Meyer S, Sahm H (2006) High-yield 5-keto-d-gluconic acid formation is mediated by soluble and membrane-bound gluconate-5-dehydrogenases of Gluconobacter oxydans. Appl Microbiol Biotechnol 73:443–451
Park KW, Kim KJ, Howard AJ, Stark BC, Webster DA (2002) Vitreoscilla hemoglobin binds to subunit I of cytochrome bo ubiquinol oxidases. J Biol Chem 277:33334–33337
Prust C, Hoffmeister M, Liesegang H, Wiezer A, Fricke WF (2005) Complete genome sequence of the acetic acid bacterium Gluconobacter oxydans. Nat Biotechnol 23:195–200
Saito Y, Ishii Y, Hayashi H, Imao Y, Akashi T, Yoshikawa K, Noguchi Y, Soeda S, Yoshida M, Niwa M, Hosoda J, Shimomura K (1997) Cloning of genes coding for l-sorbose and l-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-l-gulonate, a precursor of l-ascorbic acid in a recombinant G. oxydans strain. Appl Environ Microbiol 63:454–460
Wei XX, Chen GQ (2008) Applications of the VHb gene vgb for improved microbial fermentation processes. Methods Enzymol 436:269–283
Yang XP, Wei LJ, Lin JP, Yin B, Wei DZ (2008) Membrane-bound pyrroloquinoline quinone-dependent dehydrogenase in Gluconobacter oxydans M5, responsible for product of 6-(2-hydroxyethyl) amino-6-deoxy-l-Sorbose. Appl Environ Microbiol 74:5250–5253
Yu HM, Shi Y, Zhang YP, Yang SL, Shen ZY (2002) Effect of Vitreoscilla hemoglobin biosynthesis in Escherichia coli on production of poly (β-hydroxybutyrate) and fermentative parameters. FEMS Microbiol Lett 214:223–227
Zhang L, Li YJ, Wang ZN, Xia Y, Chen WS, Tang KX (2007) Recent developments and future prospects of Vitreoscilla hemoglobin application in metabolic engineering. Biotechnol Adv 25:123–136
Acknowledgments
This work was financially supported by the National Key Basic Research Development Program of China (“973” Program, No.2009CB724703), and the National Special Fund for State Key Laboratory of Bioreactor Engineering, Grant No. 2060204.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Li, M., Wu, J., Lin, J. et al. Expression of Vitreoscilla Hemoglobin Enhances Cell Growth and Dihydroxyacetone Production in Gluconobacter oxydans . Curr Microbiol 61, 370–375 (2010). https://doi.org/10.1007/s00284-010-9621-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00284-010-9621-6