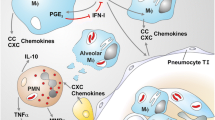
Abstract
Tuberculosis (TB) remains one of the greatest threats to human health. The causative bacterium, Mycobacterium tuberculosis (Mtb), is acquired by the respiratory route. It is exquisitely human adapted and a prototypic intracellular pathogen of macrophages, with alveolar macrophages (AMs) being the primary conduit of infection and disease. The outcome of primary infection is most often a latently infected healthy human host, in whom the bacteria are held in check by the host immune response. Such individuals can develop active TB later in life with impairment in the immune system. In contrast, in a minority of infected individuals, the host immune response fails to control the growth of bacilli, and progressive granulomatous disease develops, facilitating spread of the bacilli via infectious aerosols coughed out into the environment and inhaled by new hosts. The molecular details of the Mtb–macrophage interaction continue to be elucidated. However, it is clear that a number of complex processes are involved at the different stages of infection that may benefit either the bacterium or the host. Macrophages demonstrate tremendous phenotypic heterogeneity and functional plasticity which, depending on the site and stage of infection, facilitate the diverse outcomes. Moreover, host responses vary depending on the specific characteristics of the infecting Mtb strain. In this chapter, we describe a contemporary view of the behavior of AMs and their interaction with various Mtb strains in generating unique immunologic lung-specific responses.
Similar content being viewed by others
References
World Health Organization (2011) Global tuberculosis control. WHO report 2011. WHO, Geneva
Frieden TR, Sterling TR, Munsiff SS, Watt CJ, Dye C (2003) Tuberculosis. Lancet 362:887–899
Nuermberger E, Bishai WR, Grosset JH (2004) Latent tuberculosis infection. Semin Respir Crit Care Med 25:317–336
Murray CJL, Lopez AD (1996) The global burden of disease: a comprehensive assessment of mortality and disability from diseases, injuries and risk factors in 1990 and projected to 2020. In: Murray CJL, Lopez AD (eds) The Harvard School of Public Health on behalf of the World Health Organization and The World Bank. Harvard University Press, Cambridge, pp 1–27
Hume DA, Ross IL, Himes SR, Sasmono RT, Wells CA et al (2002) The mononuclear phagocyte system revisited. J Leukoc Biol 72:621–627
Van FR, Cohn ZA (1968) The origin and kinetics of mononuclear phagocytes. J Exp Med 128:415–435
Ebert RH, Florey HW (1939) The extravascular development of the monocyte observed in vivo. Br J Exp Path 20:342–356
Van FR, Diesselhoff-den Dulk MC, Mattie H (1973) Quantitative study on the production and kinetics of mononuclear phagocytes during an acute inflammatory reaction. J Exp Med 138:1314–1330
Kaisho T, Akira S (2000) Critical roles of Toll-like receptors in host defense. Crit Rev Immunol 20:393–405
Austyn JM, Gordon S (1981) F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11:805–815
Dijkstra CD, Van VE, Dopp EA, van der Lelij AA, Kraal G (1985) Marginal zone macrophages identified by a monoclonal antibody: characterization of immuno- and enzyme-histochemical properties and functional capacities. Immunology 55:23–30
Kraal G, Janse M (1986) Marginal metallophilic cells of the mouse spleen identified by a monoclonal antibody. Immunology 58:665–669
Kaplan G, Gaudernack G (1982) In vitro differentiation of human monocytes. Differences in monocyte phenotypes induced by cultivation on glass or on collagen. J Exp Med 156:1101–1114
Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M et al (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661
Gordon S, Taylor PR (2005) Monocyte and macrophage heterogeneity. Nat Rev Immunol 5:953–964
Sica A, Mantovani A (2012) Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122:787–795
Liddiard K, Rosas M, Davies LC, Jones SA, Taylor PR (2011) Macrophage heterogeneity and acute inflammation. Eur J Immunol 41:2503–2508
Li J, Pritchard DK, Wang X, Park DR, Bumgarner RE et al (2007) cDNA microarray analysis reveals fundamental differences in the expression profiles of primary human monocytes, monocyte-derived macrophages, and alveolar macrophages. J Leukoc Biol 81:328–335
Strauss-Ayali D, Conrad SM, Mosser DM (2007) Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol 82:244–252
Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ (2010) Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10:453–460
Grage-Griebenow E, Flad HD, Ernst M (2001) Heterogeneity of human peripheral blood monocyte subsets. J Leukoc Biol 69:11–20
Chow A, Brown BD, Merad M (2011) Studying the mononuclear phagocyte system in the molecular age. Nat Rev Immunol 11:788–798
Erwig LP, Kluth DC, Walsh GM, Rees AJ (1998) Initial cytokine exposure determines function of macrophages and renders them unresponsive to other cytokines. J Immunol 161:1983–1988
Stout RD, Suttles J (2004) Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol 76:509–513
Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B et al (2005) Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol 142:481–489
Biswas SK, Sica A, Lewis CE (2008) Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol 180:2011–2017
Stout RD, Watkins SK, Suttles J (2009) Functional plasticity of macrophages: in situ reprogramming of tumor-associated macrophages. J Leukoc Biol 86:1105–1109
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737
Taylor PR, Martinez-Pomares L, Stacey M, Lin HH, Brown GD et al (2005) Macrophage receptors and immune recognition. Annu Rev Immunol 23:901–944
Gordon S (2003) Alternative activation of macrophages. Nat Rev Immunol 3:23–35
Mosser DM (2003) The many faces of macrophage activation. J Leukoc Biol 73:209–212
Goerdt S, Orfanos CE (1999) Other functions, other genes: alternative activation of antigen-presenting cells. Immunity 10:137–142
Bogdan C (2001) Nitric oxide and the immune response. Nat Immunol 2:907–916
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604
Edwards JP, Zhang X, Frauwirth KA, Mosser DM (2006) Biochemical and functional characterization of three activated macrophage populations. J Leukoc Biol 80:1298–1307
Nair MG, Du Y, Perrigoue JG, Zaph C, Taylor JJ et al (2009) Alternatively activated macrophage-derived RELM-{alpha} is a negative regulator of type 2 inflammation in the lung. J Exp Med 206:937–952
Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL et al (2003) Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112:197–208
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H et al (2008) “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 205:1261–1268
Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A et al (2000) Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol 164:762–767
Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P et al (2009) Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer 125:367–373
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR et al (2011) CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331:1612–1616
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Helming L, Gordon S (2007) The molecular basis of macrophage fusion. Immunobiology 212:785–793
Russell DG, Cardona PJ, Kim MJ, Allain S, Altare F (2009) Foamy macrophages and the progression of the human tuberculosis granuloma. Nat Immunol 10:943–948
Mack U, Migliori GB, Sester M, Rieder HL, Ehlers S et al (2009) LTBI: latent tuberculosis infection or lasting immune responses to M. tuberculosis? A TBNET consensus statement. Eur Respir J 33:956–973
Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN et al (2003) Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun 71:7099–7108
Zhang P, Summer WR, Bagby GJ, Nelson S (2000) Innate immunity and pulmonary host defense. Immunol Rev 173:39–51
Carlson TK, Brooks M, Meyer D, Henning L et al (2010) Pulmonary innnate immunity: soluble and cellular host defenses of the lung. In: Marsh C, Tridandapani S, Piper M (eds) Regulation of innate immune function. Transworld Research Network, Kerala, pp 165–211
Zaas AK, Schwartz DA (2005) Innate immunity and the lung: defense at the interface between host and environment. Trends Cardiovasc Med 15:195–202
Fels A, Cohn ZA (1986) The alveolar macrophage. J Appl Physiol 60:353–369
Gardai SJ, Xiao YQ, Dickinson M, Nick JA, Voelker DR et al (2003) By binding SIRPalpha or calreticulin/CD91, lung collectins act as dual function surveillance molecules to suppress or enhance inflammation. Cell 115:13–23
Crouch E, Wright JR (2001) Surfactant proteins A and D and pulmonary host defense. Annu Rev Physiol 63:521–554
Crowther JE, Kutala VK, Kuppusamy P, Ferguson JS, Beharka AA et al (2004) Pulmonary surfactant protein a inhibits macrophage reactive oxygen intermediate production in response to stimuli by reducing NADPH oxidase activity. J Immunol 172:6866–6874
Williams MC (2003) Alveolar type I cells: molecular phenotype and development. Annu Rev Physiol 65:669–695
Gordon SB, Read RC (2002) Macrophage defences against respiratory tract infections. Br Med Bull 61:45–61
Suzuki T, Chow CW, Downey GP (2008) Role of innate immune cells and their products in lung immunopathology. Int J Biochem Cell Biol 40:1348–1361
Bitterman PB, Saltzman LE, Adelberg S, Ferrans VJ, Crystal RG (1984) Alveolar macrophage replication. One mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest 74:460–469
Lambrecht BN (2006) Alveolar macrophage in the driver’s seat. Immunity 24:366–368
Schlesinger LS, Azad AK, Torrelles JB, Roberts E, Vergne I et al (2008) Determinants of phagocytosis, phagosome biogenesis and autophagy for Mycobacterium tuberculosis. In: Kaufmann SHE, Britton WJ (eds) Handbook of tuberculosis. Immunology and cell biology. Wiley-VCH, Weinheim, pp 1–22
Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK et al (2010) Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol 185:929–942
Standiford TJ, Keshamouni VG, Reddy RC (2005) Peroxisome proliferator-activated receptor-{gamma} as a regulator of lung inflammation and repair. Proc Am Thorac Soc 2:226–231
Hoidal JR, Schmeling D, Peterson PK (1981) Phagocytosis, bacterial killing, and metabolism by purified human lung phagocytes. J Infect Dis 144:61–71
Roth MD, Golub SH (1993) Human pulmonary macrophages utilize prostaglandins and transforming growth factor b1 to suppress lymphocyte activation. J Leukocyte Biol 53:366–371
Lyons CR, Ball EJ, Toews GB, Weissler JC, Stastny P et al (1986) Inability of human alveolar macrophages to stimulate resting T cells correlates with decreased antigen-specific T cell-macrophage binding. J Immunol 137:1173–1180
Martinez FO, Helming L, Gordon S (2009) Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol 27:451–483
Holt PG (1979) Alveolar macrophages. III. Studies on the mechanisms of inhibition of T-cell proliferation. Immunology 37:437–445
Lipscomb MF, Lyons CR, Nunez G, Ball EJ, Stastny P et al (1986) Human alveolar macrophages: HLA-DR-positive macrophages that are poor stimulators of a primary mixed leukocyte reaction. J Immunol 136:497–504
Nguyen BY, Peterson PK, Verbrugh HA, Quie PG, Hoidal JR (1982) Differences in phagocytosis and killing by alveolar macrophages from humans, rabbits, rats, and hamsters. Infect Immun 36:504–509
Wolter NJ, Kunkel SL, Lynch JP III, Ward PA (1983) Production of cyclooxygenase products by alveolar macrophages in pulmonary sarcoidosis. Chest 83:79S–81S
Wewers MD, Rennard SI, Hance AJ, Bitterman PB, Crystal RG (1984) Normal human alveolar macrophages obtained by bronchoalveolar lavage have a limited capacity to release interleukin-1. J Clin Invest 74:2208–2218
Oren R, Farnham AE, Saito K, Milofsky E, Karnovsky ML (1963) Metabolic patterns in three types of phagocytizing cells. J Cell Biol 17:487–501
Munder M, Eichmann K, Modolell M (1998) Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. J Immunol 160:5347–5354
Suzuki K, Suda T, Naito T, Ide K, Chida K et al (2005) Impaired toll-like receptor 9 expression in alveolar macrophages with no sensitivity to CpG DNA. Am J Respir Crit Care Med 171:707–713
Monick MM, Carter AB, Gudmundsson G, Geist LJ, Hunninghake GW (1998) Changes in PKC isoforms in human alveolar macrophages compared with blood monocytes. Am J Physiol Lung Cell Mol Physiol 19:L389–L397
Jiang C, Ting AT, Seed B (1998) PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature 391:82–86
Ricote M, Li AC, Willson TM, Kelly CJ, Glass CK (1998) The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature 391:79–82
von Knethen A, Brune B (2002) Activation of peroxisome proliferator-activated receptor gamma by nitric oxide in monocytes/macrophages down-regulates p47phox and attenuates the respiratory burst. J Immunol 169:2619–2626
Beharka AA, Gaynor CD, Kang BK, Voelker DR, McCormack FX et al (2002) Pulmonary surfactant protein A up-regulates activity of the mannose receptor, a pattern recognition receptor expressed on human macrophages. J Immunol 169:3565–3573
Kuronuma K, Sano H, Kato K, Kudo K, Hyakushima N et al (2004) Pulmonary surfactant protein A augments the phagocytosis of Streptococcus pneumoniae by alveolar macrophages through a casein kinase 2-dependent increase of cell surface localization of scavenger receptor A. J Biol Chem 279:21421–21430
Henning LN, Azad AK, Parsa KV, Crowther JE, Tridandapani S et al (2008) Pulmonary surfactant protein A regulates TLR expression and activity in human macrophages. J Immunol 180:7847–7858
Wright JR (2005) Immunoregulatory functions of surfactant proteins. Nat Rev Immunol 5:58–68
Ferguson JS, Martin JL, Azad AK, McCarthy TR, Kang PB et al (2006) Surfactant protein D increases fusion of Mycobacterium tuberculosis-containing phagosomes with lysosomes in human macrophages. Infect Immun 74:7005–7009
Ferguson JS, Voelker DR, McCormack FX, Schlesinger LS (1999) Surfactant protein D binds to Mycobacterium tuberculosis bacili and lipoarrabinomannan via carbohydrate-lectin interactions resulting in reduced phagocytosis of the bacteria by macrophages. J Immunol 163:312–321
Kahnert A, Seiler P, Stein M, Bandermann S, Hahnke K et al (2006) Alternative activation deprives macrophages of a coordinated defense program to Mycobacterium tuberculosis. Eur J Immunol 36:631–647
Schafer G, Jacobs M, Wilkinson RJ, Brown GD (2009) Non-opsonic recognition of Mycobacterium tuberculosis by phagocytes. J Innate Immun 1:231–243
Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124:783–801
Takeuchi O, Akira S (2010) Pattern recognition receptors and inflammation. Cell 140:805–820
Wileman TE, Lennartz MR, Stahl PD (1986) Identification of the macrophage mannose receptor as a 175-kDa membrane protein. Proc Natl Acad Sci USA 83:2501–2505
McGreal EP, Miller JL, Gordon S (2005) Ligand recognition by antigen-presenting cell C-type lectin receptors. Curr Opin Immunol 17:18–24
Stahl PD, Ezekowitz RA (1998) The mannose receptor is a pattern recognition receptor involved in host defense. Curr Opin Immunol 10:50–55
Stahl PD (1990) The macrophage mannose receptor: current status. Am J Respir Cell Mol Biol 2:317–318
Speert DP, Silverstein SC (1985) Phagocytosis of unopsonized zymosan by human monocyte-derived macrophages: Maturation and inhibition by mannan. J Leukocyte Biol 38:655–658
Stahl PD (1992) The mannose receptor and other macrophage lectins. Curr Opin Immunol 4:49–52
Martinez-Pomares L, Linehan SA, Taylor PR, Gordon S (2001) Binding properties of the mannose receptor. Immunobiology 204:527–535
Lee SJ, Evers S, Roeder D, Parlow AF, Risteli J et al (2002) Mannose receptor-mediated regulation of serum glycoprotein homeostasis. Science 295:1901
Medzhihtov R, Janeway C Jr (2000) Innate Immunity. N Engl J Med 343:338–344
Torrelles JB, Schlesinger LS (2010) Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 90:84–93
Schlesinger LS, Kaufman TM, Iyer S, Hull SR, Marciando LK (1996) Differences in mannose receptor-mediated uptake of lipoarabinomannan from virulent and attenuated strains of Mycobacterium tuberculosis by human macrophages. J Immunol 157:4568–4575
Torrelles JB, Knaup R, Kolareth A, Slepushkina T, Kaufman TM et al (2008) Identification of Mycobacterium tuberculosis clinical isolates with altered phagocytosis by human macrophages due to a truncated lipoarabinomannan. J Biol Chem 283:31417–31428
Schlesinger LS, Hull SR, Kaufman TM (1994) Binding of the terminal mannosyl units of lipoarabinomannan from a virulent strain of Mycobacterium tuberculosis to human macrophages. J Immunol 152:4070–4079
Torrelles JB, Azad AK, Schlesinger LS (2006) Fine discrimination in the recognition of individual species of phosphatidyl-myo-inositol mannosides from Mycobacterium tuberculosis by C-type lectin pattern recognition receptors. J Immunol 177:1805–1816
Chieppa M, Bianchi G, Doni A, Del Prete A, Sironi M et al (2003) Cross-linking of the mannose receptor on monocyte-derived dendritic cells activates an anti-inflammatory immunosuppressive program. J Immunol 171:4552–4560
Nigou J, Zelle-Rieser C, Gilleron M, Thurnher M, Puzo G (2001) Mannosylated liparabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol 166:7477–7485
Astarie-Dequeker C, N’Diaye EN, Le Cabec V, Rittig MG, Prandi J et al (1999) The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect Immun 67:469–477
Aderem A, Underhill DM (1999) Mechanisms of phagocytosis in macrophages. Annu Rev Immunol 17:593–623
Kang BK, Azad AK, Torrelles JB, Kaufman TM, Beharka AA et al (2005) The human macrophage mannose receptor directs Mycobacterium tuberculosis lipoarabinomannan-mediated phagosome biogenesis. J Exp Med 202:987–999
Singh CR, Moulton RA, Armitige LY, Bidani A, Snuggs M et al (2006) Processing and presentation of a mycobacterial antigen 85B epitope by murine macrophages is dependent on the phagosomal acquisition of vacuolar proton ATPase and in situ activation of cathepsin D. J Immunol 177:3250–3259
Sturgill-Koszycki S, Schlesinger PH, Chakraborty P, Haddix PL, Collins HL et al (1994) Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263:678–681
Prigozy TI, Sieling PA, Clemens D, Stewart PL, Behar SM et al (1997) The mannose receptor delivers lipoglycan antigens to endosomes for presentation to T cells by CD1b molecules. Immunity 6:187–197
van de Veerdonk FL, Marijnissen RJ, Kullberg BJ, Koenen HJ, Cheng SC et al (2009) The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5:329–340
Martinez-Pomares L, Kosco-Vilbois M, Darley E, Tree P, Herren S et al (1996) Fc chimeric protein containing the cysteine-rich domain of the murine mannose receptor binds to macrophages from splenic marginal zone and lymph node subcapsular sinus and to germinal centers. J Exp Med 184:1927–1937
Martinez-Pomares L, Mahoney JA, Kaposzta R, Linehan SA, Stahl PD et al (1998) A functional soluble form of the murine mannose receptor is produced by macrophages in vitro and is present in mouse serum. J Biol Chem 273:23376–23380
Linehan SA, Martiniz-Pomares L, Stahl PD, Gordon S (1999) Mannose receptor and its putative ligands in normal murine lymphoid and nonlymphoid organs: in situ expression of mannose receptor by selected macrophages, endothelial cells, perivascular microglia, and mesangial cells, but not dendritic cells. J Exp Med 189:1961–1972
Engering AJ, Cella M, Fluitsma DM, Hoefsmit EC, Lanzavecchia A et al (1997) Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv Exp Med Biol 417:183–187
Tan MC, Mommaas AM, Drijfhout JW, Jordens R, Onderwater JJ et al (1997) Mannose receptor mediated uptake of antigens strongly enhances HLA-class II restricted antigen presentation by cultured dendritic cells. Adv Exp Med Biol 417:171–174
Berney C, Herren S, Power CA, Gordon S, Martinez-Pomares L et al (1999) A member of the dendritic cell family that enters B cell follicles and stimulates primary antibody responses identified by a mannose receptor fusion protein. J Exp Med 190:851–860
McNally AK, DeFife KM, Anderson JM (1996) Interleukin-4-induced macrophage fusion is prevented by inhibitors of mannose receptor activity. Am J Pathol 149:975–985
Mitchell DA, Fadden AJ, Drickamer K (2001) A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J Biol Chem 276:28939–28945
Feinberg H, Mitchell DA, Drickamer K, Weis WI (2001) Structural basis for selective recognition of oligosaccharides by DC-SIGN and DC-SIGNR. Science 294:2163–2166
Figdor CG, Van Kooyk Y, Adema GJ (2002) C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol 2:77–84
Geijtenbeek TB, Torensma R, Van Vliet SJ, van Duijnhoven GC, Adema GJ et al (2000) Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575–585
Bleijs DA, Geijtenbeek TB, Figdor CG, van Kooyk Y (2001) DC-SIGN and LFA-1: a battle for ligand. Trends Immunol 22:457–463
van Kooyk Y, Geijtenbeek TB (2003) DC-SIGN: escape mechanism for pathogens. Nat Rev Immunol 3:697–709
Tailleux L, Pham-Thi N, Bergeron-Lafaurie A, Herrmann JL, Charles P et al (2005) DC-SIGN induction in alveolar macrophages defines privileged target host cells for mycobacteria in patients with tuberculosis. PLoS Med 2:e381
Puig-Kroger A, Serrano-Gomez D, Caparros E, Dominguez-Soto A, Relloso M et al (2004) Regulated expression of the pathogen receptor dendritic cell-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin in THP-1 human leukemic cells, monocytes, and macrophages. J Biol Chem 279:25680–25688
Geijtenbeek TB, Van Vliet SJ, Koppel EA, Sanchez-Hernandez M, Vandenbroucke-Grauls CM et al (2003) Mycobacteria target DC-SIGN to suppress dendritic cell function. J Exp Med 197:7–17
Bodnar KA, Serbina NV, Flynn JL (2001) Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun 69:800–809
Tailleux L, Schwartz O, Herrmann JL, Pivert E, Jackson M et al (2003) DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J Exp Med 197:121–127
Engering A, Geijtenbeek TB, Van Vliet SJ, Wijers M, van Liempt E et al (2002) The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol 168:2118–2126
Taylor PR, Brown GD, Reid DM, Willment JA, Martinez-Pomares L et al (2002) The beta-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J Immunol 169:3876–3882
Abbas AK, Lichtman AH (2005) Cellular and molecular immunology, 5th edn. Saunders, Philadelphia
Lee MS, Kim YJ (2007) Signaling pathways downstream of pattern-recognition receptors and their cross talk. Annu Rev Biochem 76:447–480
Yadav M, Schorey JS (2006) The {beta}-glucan receptor Dectin-1 functions together with TLR2 to mediate macrophage activation by mycobacteria. Blood 108:3168–3175
Rothfuchs AG, Bafica A, Feng CG, Egen JG, Williams DL et al (2007) Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J Immunol 179:3463–3471
van de Veerdonk FL, Teirlinck AC, Kleinnijenhuis J, Kullberg BJ, van Crevel R et al (2010) Mycobacterium tuberculosis induces IL-17A responses through TLR4 and dectin-1 and is critically dependent on endogenous IL-1. J Leukoc Biol 88:227–232
Zenaro E, Donini M, Dusi S (2009) Induction of Th1/Th17 immune response by Mycobacterium tuberculosis: role of dectin-1, mannose receptor, and DC-SIGN. J Leukoc Biol 86:1393–1401
Yamasaki S, Ishikawa E, Sakuma M, Hara H, Ogata K et al (2008) Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat Immunol 9:1179–1188
Yamasaki S, Matsumoto M, Takeuchi O, Matsuzawa T, Ishikawa E et al (2009) C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc Natl Acad Sci U S A 106:1897–1902
Ishikawa E, Ishikawa T, Morita YS, Toyonaga K, Yamada H et al (2009) Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J Exp Med 206:2879–2888
Heitmann L, Schoenen H, Ehlers S, Lang R, Holscher C (2013) Mincle is not essential for controlling Mycobacterium tuberculosis infection. Immunobiology 218:506–516
Myones BL, Dalzell JG, Hogg N, Ross GD (1988) Neutrophil and monocyte cell surface p150,955 has iC3b-receptor (CR4) activity resembling CR3. J Clin Invest 82:640–651
Arnaout MA (1990) Structure and function of the leukocyte adhesion molecules CD11/CD18. Blood 75:1037–1050
Schlesinger LS, Bellinger-Kawahara CG, Payne NR, Horwitz MA (1990) Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J Immunol 144:2771–2780
Schlesinger LS (1993) Macrophage phagocytosis of virulent but not attenuated strains of Mycobacterium tuberculosis is mediated by mannose receptors in addition to complement receptors. J Immunol 150:2920–2930
Ferguson JS, Weis JJ, Martin JL, Schlesinger LS (2004) Complement protein C3 binding to Mycobacterium tuberculosis is initiated by the classical pathway in human bronchoalveolar lavage fluid. Infect Immun 72:2564–2573
Cywes C, Hoppe HC, Daffe M, Ehlers MRW (1997) Nonopsonic binding of Mycobacterium tuberculosis to complement receptor type 3 is mediated by capsular polysaccharides and is strain dependent. Infect Immun 65:4258–4266
Villeneuve C, Gilleron M, Maridonneau-Parini I, Daffe M, Astarie-Dequeker C et al (2005) Mycobacteria use their surface-exposed glycolipids to infect human macrophages through a receptor-dependent process. J Lipid Res 46:475–483
Melo MD, Catchpole IR, Haggar G, Stokes RW (2000) Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell Immunol 205:13–23
Hu C, Mayadas-Norton T, Tanaka K, Chan J, Salgame P (2000) Mycobacterium tuberculosis infection in complement receptor 3-deficient mice. J Immunol 165:2596–2602
Armstrong JA, Hart PD (1975) Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli: reversal of the usual nonfusion pattern and observations on bacterial survial. J Exp Med 142:1–16
Basu S, Fenton MJ (2004) Toll-like receptors: function and roles in lung disease. Am J Physiol Lung Cell Mol Physiol 286:L887–L892
Krutzik SR, Modlin RL (2004) The role of Toll-like receptors in combating mycobacteria. Semin Immunol 16:35–41
Hayashi F, Means TK, Luster AD (2003) Toll-like receptors stimulate human neutrophil function. Blood 102:2660–2669
Dasari P, Nicholson IC, Hodge G, Dandie GW, Zola H (2005) Expression of toll-like receptors on B lymphocytes. Cell Immunol 236:140–145
Kodowaki N, Ho S, Antonenko S, de Waal MR, Kastelein RA et al (2001) Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med 194:863–869
Armstrong L, Medford AR, Uppington KM, Robertson J, Witherden IR et al (2004) Expression of functional toll-like receptor-2 and −4 on alveolar epithelial cells. Am J Respir Cell Mol Biol 31:241–245
Medzhitov R, Preston-Hurlburt P, Janeway CA Jr (1997) A human homologue of the Drosophia Toll protein signals activation of adaptive immunity. Nature 388:394–397
Rock FL, Hardiman G, Timans JC, Kastelein RA, Bazan JF (1998) A family of human receptors structurally related to Drosophila Toll. Proc Natl Acad Sci U S A 95:588–593
Brightbill HD, Libraty DH, Krutzik SR, Yang RB, Belisle JT et al (1999) Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285:732–736
Trinchieri G, Sher A (2007) Cooperation of Toll-like receptor signals in innate immune defence. Nat Rev Immunol 7:179–190
Poltorak A, He X, Smirnova I, Liu MY, Van HC et al (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085–2088
Malhotra R, Thiel S, Reid KBM, Sim RB (1990) Human leukocyte Clq receptor binds other soluble proteins with collagen domains. J Exp Med 172:955–959
Kobayashi K, Hernandez LD, Galan JE, Janeway CA Jr, Medzhitov R et al (2002) IRAK-M is a negative regulator of Toll-like receptor signaling. Cell 110:191–202
Nguyen HA, Rajaram MV, Meyer DA, Schlesinger LS (2012) Pulmonary surfactant protein A and surfactant lipids upregulate IRAK-M, a negative regulator of TLR-mediated inflammation in human macrophages. Am J Physiol Lung Cell Mol Physiol 303:L608–L616
Yamamoto M, Takeda K, Akira S (2004) TIR domain-containing adaptors define the specificity of TLR signaling. Mol Immunol 40:861–868
Cambi A, Koopman M, Figdor CG (2005) How C-type lectins detect pathogens. Cell Microbiol 7:481–488
Kawai T, Akira S (2011) Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650
Kawai T, Akira S (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11:373–384
Jo EK (2008) Mycobacterial interaction with innate receptors: TLRs, C-type lectins, and NLRs. Curr Opin Infect Dis 21:279–286
Rosenberg PS, Che A, Chen BE (2006) Multiple hypothesis testing strategies for genetic case–control association studies. Stat Med 25:3134–3149
Heldwein KA, Fenton MJ (2002) The role of toll-like receptors in immunity against mycobacterial infection. Microbes Infect 4:937–944
Quesniaux V, Fremond C, Jacobs M, Parida S, Nicolle D et al (2004) Toll-like receptor pathways in the immune responses to mycobacteria. Microbes Infect 6:946–959
Jo EK, Yang CS, Choi CH, Harding CV (2007) Intracellular signalling cascades regulating innate immune responses to Mycobacteria: branching out from Toll-like receptors. Cell Microbiol 9:1087–1098
Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K et al (2002) Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169:10–14
Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT et al (1999) Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol 163:3920–3927
Lopez M, Sly LM, Luu Y, Young D, Cooper H et al (2003) The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J Immunol 170:2409–2416
Dao DN, Kremer L, Guerardel Y, Molano A, Jacobs WR Jr et al (2004) Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect Immun 72:2067–2074
Vergne I, Fratti RA, Hill PJ, Chua J, Belisle J et al (2004) Mycobacterium tuberculosis phagosome maturation arrest: mycobacterial phosphatidylinositol analog phosphatidylinositol mannoside stimulates early endosomal fusion. Mol Biol Cell 15:751–760
Palecanda A, Kobzik L (2001) Receptors for unopsonized particles: the role of alveolar macrophage scavenger receptors. Curr Mol Med 1:589–595
Krieger M (1992) Molecular flypaper and atherosclerosis: structure of the macrophage scavenger receptor. Trends Biochem Sci 17:141–146
Postlethwait EM (2007) Scavenger receptors clear the air. J Clin Invest 117:601–604
Bowdish DM, Sakamoto K, Kim MJ, Kroos M, Mukhopadhyay S et al (2009) MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog 5:e1000474
Pugin J, Heumann D, Tomasz A, Kravchenko VV, Akamatsu Y et al (1994) CD14 is a pattern recognition receptor. Immunity 1:509–516
Dziarski R (2003) Recognition of bacterial peptidoglycan by the innate immune system. Cell Mol Life Sci 60:1793–1804
Fujihara M, Muroi M, Tanamoto K, Suzuki T, Azuma H et al (2003) Molecular mechanisms of macrophage activation and deactivation by lipopolysaccharide: roles of the receptor complex. Pharmacol Ther 100:171–194
Flo TH, Ryan L, Kilaas L, Skjak-Braek G, Ingalls RR et al (2000) Involvement of CD14 and beta2-integrins in activating cells with soluble and particulate lipopolysaccharides and mannuronic acid polymers. Infect Immun 68:6770–6776
Bernardo J, Billingslea AM, Blumenthal RL, Seetoo KF, Simons ER et al (1998) Differential responses of human mononuclear phagocytes to mycobacterial lipoarabinomannans: role of CD14 and the mannose receptor. Infect Immun 66:28–35
Peterson PK, Gekker G, Hu S, Sheng WS, Anderson WR et al (1995) CD14 receptor-mediated uptake of nonopsonized Mycobacterium tuberculosis by human microglia. Infect Immun 63:1598–1602
Shams H, Wizel B, Lakey DL, Samten B, Vankayalapati R et al (2003) The CD14 receptor does not mediate entry of Mycobacterium tuberculosis into human mononuclear phagocytes. FEMS Immunol Med Microbiol 36:63–69
Inohara C, McDonald C, Nunez G (2005) NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu Rev Biochem 74:355–383
Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM et al (2008) The NLR gene family: a standard nomenclature. Immunity 28:285–287
Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S et al (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 276:4812–4818
Brooks MN, Rajaram MV, Azad AK, Amer AO, Valdivia-Arenas MA et al (2011) NOD2 controls the nature of the inflammatory response and subsequent fate of Mycobacterium tuberculosis and M. bovis BCG in human macrophages. Cell Microbiol 13:402–418
Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F et al (2003) Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem 278:5509–5512
Chen CM, Gong Y, Zhang M, Chen JJ (2004) Reciprocal cross-talk between Nod2 and TAK1 signaling pathways. J Biol Chem 279:25876–25882
Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S et al (2008) A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A 105:7803–7808
Netea MG, Sutmuller R, Hermann C, Van der Graaf CA, Van der Meer JW et al (2004) Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol 172:3712–3718
Netea MG, Ferwerda G, de Jong DJ, Jansen T, Jacobs L et al (2005) Nucleotide-binding oligomerization domain-2 modulates specific TLR pathways for the induction of cytokine release. J Immunol 174:6518–6523
Uehara A, Sugawara Y, Kurata S, Fujimoto Y, Fukase K et al (2005) Chemically synthesized pathogen-associated molecular patterns increase the expression of peptidoglycan recognition proteins via toll-like receptors, NOD1 and NOD2 in human oral epithelial cells. Cell Microbiol 7:675–686
van Heel DA, Ghosh S, Hunt KA, Mathew CG, Forbes A et al (2005) Synergy between TLR9 and NOD2 innate immune responses is lost in genetic Crohn’s disease. Gut 54:1553–1557
Watanabe T, Kitani A, Murray PJ, Strober W (2004) NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol 5:800–808
Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ et al (2006) Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity 25:473–485
Wolfert MA, Murray TF, Boons GJ, Moore JN (2002) The origin of the synergistic effect of muramyl dipeptide with endotoxin and peptidoglycan. J Biol Chem 277:39179–39186
Yang S, Tamai R, Akashi S, Takeuchi O, Akira S et al (2001) Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun 69:2045–2053
Girardin SE, Tournebize R, Mavris M, Page AL, Li X et al (2001) CARD4/Nod1 mediates NF-kappaB and JNK activation by invasive Shigella flexneri. EMBO Rep 2:736–742
Marriott I, Rati DM, McCall SH, Tranguch SL (2005) Induction of Nod1 and Nod2 intracellular pattern recognition receptors in murine osteoblasts following bacterial challenge. Infect Immun 73:2967–2973
Opitz B, Forster S, Hocke AC, Maass M, Schmeck B et al (2005) Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ Res 96:319–326
Travassos LH, Carneiro LA, Girardin SE, Boneca IG, Lemos R et al (2005) Nod1 participates in the innate immune response to Pseudomonas aeruginosa. J Biol Chem 280:36714–36718
Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE et al (2004) Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 5:1166–1174
Opitz B, Puschel A, Beermann W, Hocke AC, Forster S et al (2006) Listeria monocytogenes activated p38 MAPK and induced IL-8 secretion in a nucleotide-binding oligomerization domain 1-dependent manner in endothelial cells. J Immunol 176:484–490
Opitz B, Puschel A, Schmeck B, Hocke AC, Rosseau S et al (2004) Nucleotide-binding oligomerization domain proteins are innate immune receptors for internalized Streptococcus pneumoniae. J Biol Chem 279:36426–36432
Ferwerda G, Girardin SE, Kullberg BJ, Le Bourhis L, de Jong DJ et al (2005) NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog 1:279–285
Divangahi M, Mostowy S, Coulombe F, Kozak R, Guillot L et al (2008) NOD2-deficient mice have impaired resistance to Mycobacterium tuberculosis infection through defective innate and adaptive immunity. J Immunol 181:7157–7165
Deshmukh HS, Hamburger JB, Ahn SH, McCafferty DG, Yang SR et al (2009) Critical role of NOD2 in regulating the immune response to Staphylococcus aureus. Infect Immun 77:1376–1382
Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N et al (2006) Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem 281:35217–35223
Gandotra S, Jang S, Murray PJ, Salgame P, Ehrt S (2007) Nucleotide-binding oligomerization domain protein 2-deficient mice control infection with Mycobacterium tuberculosis. Infect Immun 75:5127–5134
Reiner NE (1994) Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today 15:374–381
Bhatt K, Salgame P (2007) Host innate immune response to Mycobacterium tuberculosis. J Clin Immunol 27:347–362
Dietrich J, Doherty TM (2009) Interaction of Mycobacterium tuberculosis with the host: consequences for vaccine development. APMIS 117:440–457
Nguyen L, Pieters J (2005) The Trojan horse: survival tactics of pathogenic mycobacteria in macrophages. Trends Cell Biol 15:269–276
Deretic V, Singh S, Master S, Harris J, Roberts E et al (2006) Mycobacterium tuberculosis inhibition of phagolysosome biogenesis and autophagy as a host defence mechanism. Cell Microbiol 8:719–727
Russell DG (2001) Mycobacterium tuberculosis: here today, and here tomorrow. Nature Reviews 2:1–9
Sturgill-Koszycki S, Schaible UE, Russell DG (1996) Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J 15:6960–6968
Clemens DL, Horwitz MA (1996) The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J Exp Med 184:1349–1355
Russell DG, Dant J, Sturgill-Koszycki S (1996) Mycobacterium avium- and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J Immunol 156:4764–4773
Fratti RA, Chua J, Vergne I, Deretic V (2003) Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc Natl Acad Sci U S A 100:5437–5442
Chua J, Vergne I, Master S, Deretic V (2004) A tale of two lipids: Mycobacterium tuberculosis phagosome maturation arrest. Curr Opin Microbiol 7:71–77
Vergne I, Chua J, Deretic V (2003) Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J Exp Med 198:653–659
Kaufmann SHE, Britton WJ (eds) (2008) Handbook of tuberculosis: immunology and cell biology, 1st edn. Hoboken, Wiley-Blackwell
Malik ZA, Thompson CR, Hashimi S, Porter B, Iyer SS et al (2003) Cutting edge: Mycobacterium tuberculosis blocks Ca(2+) signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J Immunol 170:2811–2815
Fratti RA, Backer JM, Gruenberg J, Corvera S, Deretic V (2001) Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J Cell Biol 154:631–644
Kusner DJ (2005) Mechanisms of mycobacterial persistence in tuberculosis. Clin Immunol 114:239–247
Connolly SF, Kusner DJ (2007) The regulation of dendritic cell function by calcium-signaling and its inhibition by microbial pathogens. Immunol Res 39:115–127
Deretic V, Vergne I, Chua J, Master S, Singh SB et al (2004) Endosomal membrane traffic: convergence point targeted by Mycobacterium tuberculosis and HIV. Cell Microbiol 6:999–1009
Clemens DL, Lee BY, Horwitz MA (2000) Deviant expression of Rab5 on phagosomes containing the intracellular pathogens Mycobacterium tuberculosis and Legionella pneumophila is associated with altered phagosomal fate. Infect Immun 68:2671–2684
Via LE, Deretic D, Ulmer RJ, Hibler NS, Huber LA et al (1997) Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J Biol Chem 272:13326–13331
Hinchey J, Lee S, Jeon BY, Basaraba RJ, Venkataswamy MM et al (2007) Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J Clin Invest 117:2279–2288
Hou JM, D’Lima NG, Rigel NW, Gibbons HS, McCann JR et al (2008) ATPase activity of Mycobacterium tuberculosis SecA1 and SecA2 proteins and its importance for SecA2 function in macrophages. J Bacteriol 190:4880–4887
Tan T, Lee WL, Alexander DC, Grinstein S, Liu J (2006) The ESAT-6/CFP-10 secretion system of Mycobacterium marinum modulates phagosome maturation. Cell Microbiol 8:1417–1429
Walburger A, Koul A, Ferrari G, Nguyen L, Prescianotto-Baschong C et al (2004) Protein kinase G from pathogenic mycobacteria promotes survival within macrophages. Science 304:1800–1804
Chan J, Fan X, Hunter SW, Brennan PJ, Bloom BR (1991) Lipoarabinomannan, a possible virulence factor involved in persistence of Mycobacterium tuberculosis within macrophages. Infect Immun 59:1755–1761
Knutson KL, Hmama Z, Herrera-Velit P, Rochford R, Reiner NE (1997) Lipoarabinomannan of Mycobacterium tuberculosis promotes portein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. J Biol Chem 273:645–652
Kan-Sutton C, Jagannath C, Hunter RL Jr (2009) Trehalose 6,6’-dimycolate on the surface of Mycobacterium tuberculosis modulates surface marker expression for antigen presentation and costimulation in murine macrophages. Microbes Infect 11:40–48
Lee W, VanderVen BC, Fahey RJ, Russell DG (2013) Intracellular Mycobacterium tuberculosis exploits host-derived fatty acids to limit metabolic stress. J Biol Chem 288:6788–6800
Daniel J, Maamar H, Deb C, Sirakova TD, Kolattukudy PE (2011) Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog 7:e1002093
Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C et al (2008) Foamy macrophages from tuberculous patients’ granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog 4:e1000204
Welin A, Lerm M (2012) Inside or outside the phagosome? The controversy of the intracellular localization of Mycobacterium tuberculosis. Tuberculosis (Edinb) 92:113–120
Leake ES, Myrvik QN, Wright MJ (1984) Phagosomal membranes of Mycobacterium bovis BCG-immune alveolar macrophages are resistant to disruption by Mycobacterium tuberculosis H37Rv. Infect Immun 45:443–446
McDonough KA, Kress Y, Bloom BR (1993) Pathogenesis of tuberculosis: interaction of Mycobacterium tuberculosis with macrophages. Infect Immun 61:2763–2773
Myrvik QN, Leake ES, Wright MJ (1984) Disruption of phagosomal membranes of normal alveolar macrophages by the H37Rv strain of Mycobacterium tuberculosis. A correlate of virulence. Am Rev Respir Dis 129:322–328
Stamm LM, Morisaki JH, Gao LY, Jeng RL, McDonald KL et al (2003) Mycobacterium marinum escapes from phagosomes and is propelled by actin-based motility. J Exp Med 198:1361–1368
Smith J, Manoranjan J, Pan M, Bohsali A, Xu J et al (2008) Evidence for pore formation in host cell membranes by ESX-1-secreted ESAT-6 and its role in Mycobacterium marinum escape from the vacuole. Infect Immun 76:5478–5487
Hagedorn M, Rohde KH, Russell DG, Soldati T (2009) Infection by tubercular mycobacteria is spread by nonlytic ejection from their amoeba hosts. Science 323:1729–1733
van der Wel N, Hava D, Houben D, Fluitsma D, van Zon M et al (2007) M. tuberculosis and M. leprae translocate from the phagolysosome to the cytosol in myeloid cells. Cell 129:1287–1298
Houben D, Demangel C, van Ingen J, Perez J, Baldeon L et al (2012) ESX-1-mediated translocation to the cytosol controls virulence of mycobacteria. Cell Microbiol 14:1287–1298
Wong KW, Jacobs WR Jr (2011) Critical role for NLRP3 in necrotic death triggered by Mycobacterium tuberculosis. Cell Microbiol 13:1371–1384
Welin A, Eklund D, Stendahl O, Lerm M (2011) Human macrophages infected with a high burden of ESAT-6-expressing M. tuberculosis undergo caspase-1- and cathepsin B-independent necrosis. PLoS One 6:e20302
Simeone R, Bobard A, Lippmann J, Bitter W, Majlessi L et al (2012) Phagosomal rupture by Mycobacterium tuberculosis results in toxicity and host cell death. PLoS Pathog 8:e1002507
Manzanillo PS, Shiloh MU, Portnoy DA, Cox JS (2012) Mycobacterium tuberculosis activates the DNA-dependent cytosolic surveillance pathway within macrophages. Cell Host Microbe 11:469–480
Watson RO, Manzanillo PS, Cox JS (2012) Extracellular M. tuberculosis DNA targets bacteria for autophagy by activating the host DNA-sensing pathway. Cell 150:803–815
Harriff MJ, Purdy GE, Lewinsohn DM (2012) Escape from the phagosome: the explanation for MHC-I processing of mycobacterial antigens? Front Immunol 3:40
Weerdenburg EM, Peters PJ, van der Wel NN (2010) How do mycobacteria activate CD8+ T cells? Trends Microbiol 18:1–10
Guermonprez P, Saveanu L, Kleijmeer M, Davoust J, van Endert P et al (2003) ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425:397–402
Flynn JL, Chan J (2001) Immunology of tuberculosis. Annu Rev Immunol 19:93–129
Cooper AM (2009) Cell-mediated immune responses in tuberculosis. Annu Rev Immunol 27:393–422
Fenhalls G, Stevens L, Bezuidenhout J, Amphlett GE, Duncan K et al (2002) Distribution of IFN-gamma, IL-4 and TNF-alpha protein and CD8 T cells producing IL-12p40 mRNA in human lung tuberculous granulomas. Immunology 105:325–335
Herrera MT, Torres M, Nevels D, Perez-Redondo CN, Ellner JJ et al (2009) Compartmentalized bronchoalveolar IFN-gamma and IL-12 response in human pulmonary tuberculosis. Tuberculosis (Edinb) 89:38–47
Kellar KL, Gehrke J, Weis SE, Mahmutovic-Mayhew A, Davila B et al (2011) Multiple cytokines are released when blood from patients with tuberculosis is stimulated with Mycobacterium tuberculosis antigens. PLoS One 6:e26545
Unsal E, Aksaray S, Koksal D, Sipit T (2005) Potential role of interleukin 6 in reactive thrombocytosis and acute phase response in pulmonary tuberculosis. Postgrad Med J 81:604–607
Guler R, Parihar SP, Spohn G, Johansen P, Brombacher F et al (2011) Blocking IL-1alpha but not IL-1beta increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine 29:1339–1346
Zhang Y, Rom WN (1993) Regulation of the interleukin-1b (IL-1b) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol 13:3831–3837
Kaufmann SH (2001) How can immunology contribute to the control of tuberculosis? Nat Rev Immunol 1:20–30
Roach DR, Bean AG, Demangel C, France MP, Briscoe H et al (2002) TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol 168:4620–4627
Ray JC, Flynn JL, Kirschner DE (2009) Synergy between individual TNF-dependent functions determines granuloma performance for controlling Mycobacterium tuberculosis infection. J Immunol 182:3706–3717
Pompei L, Jang S, Zamlynny B, Ravikumar S, McBride A et al (2007) Disparity in IL-12 release in dendritic cells and macrophages in response to Mycobacterium tuberculosis is due to use of distinct TLRs. J Immunol 178:5192–5199
Giacomini E, Iona E, Ferroni L, Miettinen M, Fattorini L et al (2001) Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. J Immunol 166:7033–7041
Hickman SP, Chan J, Salgame P (2002) Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol 168:4636–4642
Cooper AM, Solache A, Khader SA (2007) Interleukin-12 and tuberculosis: an old story revisited. Curr Opin Immunol 19:441–447
Chen Q, Ghilardi N, Wang H, Baker T, Xie MH et al (2000) Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature 407:916–920
Trinchieri G, Pflanz S, Kastelein RA (2003) The IL-12 family of heterodimeric cytokines: new players in the regulation of T cell responses. Immunity 19:641–644
Pearl JE, Khader SA, Solache A, Gilmartin L, Ghilardi N et al (2004) IL-27 signaling compromises control of bacterial growth in mycobacteria-infected mice. J Immunol 173:7490–7496
Holscher C, Holscher A, Ruckerl D, Yoshimoto T, Yoshida H et al (2005) The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol 174:3534–3544
Robinson CM, Jung JY, Nau GJ (2012) Interferon-gamma, tumor necrosis factor, and interleukin-18 cooperate to control growth of Mycobacterium tuberculosis in human macrophages. Cytokine 60:233–241
Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J et al (2007) IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8:369–377
Khader SA, Gaffen SL, Kolls JK (2009) Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2:403–411
Lockhart E, Green AM, Flynn JL (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177:4662–4669
Sergejeva S, Ivanov S, Lotvall J, Linden A (2005) Interleukin-17 as a recruitment and survival factor for airway macrophages in allergic airway inflammation. Am J Respir Cell Mol Biol 33:248–253
Kolls JK, Linden A (2004) Interleukin-17 family members and inflammation. Immunity 21:467–476
Khader SA, Cooper AM (2008) IL-23 and IL-17 in tuberculosis. Cytokine 41:79–83
Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA et al (2011) IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol 187:5402–5407
Akira S, Taga T, Kishimoto T (1993) Interleukin-6 in biology and medicine. Adv Immunol 54:1–78
Kishimoto T, Akira S, Taga T (1992) Interleukin-6 and its receptor: a paradigm for cytokines. Science 258:593–597
Chan ED, Morris KR, Belisle JT, Hill P, Remigio LK et al (2001) Induction of inducible nitric oxide synthase-NO• by lipoarabinomannan of Mycobacterium tuberculosis is mediated by MEK1-ERK, MKK7-JNK, and NF-kB signaling pathways. Infect Immun 69:2001–2010
Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550
Hernandez-Pando R, Orozco-Esteves H, Maldonado HA, Guilar-Leon D, Vilchis-Landeros MM et al (2006) A combination of a transforming growth factor-beta antagonist and an inhibitor of cyclooxygenase is an effective treatment for murine pulmonary tuberculosis. Clin Exp Immunol 144:264–272
Yang CS, Yuk JM, Shin DM, Kang J, Lee SJ et al (2009) Secretory phospholipase A2 plays an essential role in microglial inflammatory responses to Mycobacterium tuberculosis. Glia 57:1091–1103
Sabat R (2010) IL-10 family of cytokines. Cytokine Growth Factor Rev 21:315–324
Sabat R, Grutz G, Warszawska K, Kirsch S, Witte E et al (2010) Biology of interleukin-10. Cytokine Growth Factor Rev 21:331–344
Bogdan C, Vodovotz Y, Nathan C (1991) Macrophage deactivation by interleukin 10. J Exp Med 174:1549–1555
de Waal MR, Abrams J, Bennett B, Figdor CG, De Vries JE (1991) Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med 174:1209–1220
Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A (1991) IL-10 inhibits cytokine production by activated macrophages. J Immunol 147:3815–3822
D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M et al (1993) Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med 178:1041–1048
Gruber MF, Williams CC, Gerrard TL (1994) Macrophage-colony-stimulating factor expression by anti- CD45 stimulated human monocytes is transcriptionally up- regulated by IL-1b and inhibited by IL-4 and IL-10. J Immunol 152:1354–1361
Aste-Amezaga M, Ma X, Sartori A, Trinchieri G (1998) Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J Immunol 160:5936–5944
Cunha FQ, Moncada S, Liew FY (1992) Interleukin-10 (IL-10) inhibits the induction of nitric oxide synthase by interferon-gamma in murine macrophages. Biochem Biophys Res Commun 182:1155–1159
Kuga S, Otsuka T, Niiro H, Nunoi H, Nemoto Y et al (1996) Suppression of superoxide anion production by interleukin-10 is accompanied by a downregulation of the genes for subunit proteins of NADPH oxidase. Exp Hematol 24:151–157
Moore KW, de Waal MR, Coffman RL, O’Garra A (2001) Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol 19:683–765
O’Leary S, O’Sullivan MP, Keane J (2011) IL-10 blocks phagosome maturation in Mycobacterium tuberculosis-infected human macrophages. Am J Respir Cell Mol Biol 45:172–180
Flynn JL, Chan J (2005) What’s good for the host is good for the bug. Trends Microbiol 13:98–102
Mendez-Samperio P (2008) Expression and regulation of chemokines in mycobacterial infection. J Infect 57:374–384
Saunders BM, Britton WJ (2007) Life and death in the granuloma: immunopathology of tuberculosis. Immunol Cell Biol 85:103–111
Algood HM, Chan J, Flynn JL (2003) Chemokines and tuberculosis. Cytokine Growth Factor Rev 14:467–477
Sadek MI, Sada E, Toossi Z, Schwander SK, Rich EA (1998) Chemokines induced by infection of mononuclear phagocytes with mycobacteria and present in lung alveoli during active pulmonary tuberculosis. Am J Respir Cell Mol Biol 19:513–521
Saukkonen JJ, Bazydlo B, Thomas M, Strieter RM, Keane J et al (2002) Beta-chemokines are induced by Mycobacterium tuberculosis and inhibit its growth. Infect Immun 70:1684–1693
Taub DD, Turcovski-Corrales SM, Key ML, Longo DL, Murphy WJ (1996) Chemokines and T lymphocyte activation: I. Beta chemokines costimulate human T lymphocyte activation in vitro. J Immunol 156:2095–2103
Fahey TJ III, Tracey KJ, Tekamp-Olson P, Cousens LS, Jones WG et al (1992) Macrophage inflammatory protein 1 modulates macrophage function. J Immunol 148:2764–2769
Karpus WJ, Kennedy KJ (1997) MIP-1alpha and MCP-1 differentially regulate acute and relapsing autoimmune encephalomyelitis as well as Th1/Th2 lymphocyte differentiation. J Leukoc Biol 62:681–687
Kurashima K, Mukaida N, Fujimura M, Yasui M, Nakazumi Y et al (1997) Elevated chemokine levels in bronchoalveolar lavage fluid of tuberculosis patients. Am J Respir Crit Care Med 155:1474–1477
Chensue SW, Warmington KS, Allenspach EJ, Lu B, Gerard C et al (1999) Differential expression and cross-regulatory function of RANTES during mycobacterial (type 1) and schistosomal (type 2) antigen-elicited granulomatous inflammation. J Immunol 163:165–173
Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M et al (1998) B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med 187:655–660
Jones BW, Heldwein KA, Means TK, Saukkonen JJ, Fenton MJ (2001) Differential roles of Toll-like receptors in the elicitation of proinflammatory responses by macrophages. Ann Rheum Dis 60(Suppl 3):iii6–iii12
Martin CJ, Booty MG, Rosebrock TR, Nunes-Alves C, Desjardins DM et al (2012) Efferocytosis is an innate antibacterial mechanism. Cell Host Microbe 12:289–300
Harris J, Keane J (2010) How tumour necrosis factor blockers interfere with tuberculosis immunity. Clin Exp Immunol 161:1–9
Persson YA, Blomgran-Julinder R, Rahman S, Zheng L, Stendahl O (2008) Mycobacterium tuberculosis-induced apoptotic neutrophils trigger a pro-inflammatory response in macrophages through release of heat shock protein 72, acting in synergy with the bacteria. Microbes Infect 10:233–240
Golstein P, Kroemer G (2007) Cell death by necrosis: towards a molecular definition. Trends Biochem Sci 32:37–43
Repasy T, Lee J, Marino S, Martinez N, Kirschner DE et al (2013) Intracellular bacillary burden reflects a burst size for Mycobacterium tuberculosis in vivo. PLoS Pathog 9:e1003190
Koo IC, Wang C, Raghavan S, Morisaki JH, Cox JS et al (2008) ESX-1-dependent cytolysis in lysosome secretion and inflammasome activation during mycobacterial infection. Cell Microbiol 10:1866–1878
Carlsson F, Kim J, Dumitru C, Barck KH, Carano RA et al (2010) Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog 6:e1000895
Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G et al (2010) Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol 12:1046–1063
Elkington PT, Friedland JS (2006) Matrix metalloproteinases in destructive pulmonary pathology. Thorax 61:259–266
Elkington PT, Ugarte-Gil CA, Friedland JS (2011) Matrix metalloproteinases in tuberculosis. Eur Respir J 38:456–464
Elkington PT, D’Armiento JM, Friedland JS (2011) Tuberculosis immunopathology: the neglected role of extracellular matrix destruction. Sci Transl Med 3:71ps6
Davidson JM (1990) Biochemistry and turnover of lung interstitium. Eur Respir J 3:1048–1063
Chang JC, Wysocki A, Tchou-Wong KM, Moskowitz N, Zhang YH et al (1996) Effect of Mycobacterium tuberculosis and its components on macrophages and the release of matrix metalloproteinases. Thorax 51:306–311
Rivera-Marrero CA, Schuyler W, Roser S, Ritzenthaler JD, Newburn SA et al (2002) M. tuberculosis induction of matrix metalloproteinase-9: the role of mannose and receptor-mediated mechanisms. Am J Physiol Lung Cell Mol Physiol 282:L546–L555
Elass E, Aubry L, Masson M, Denys A, Guerardel Y et al (2005) Mycobacterial lipomannan induces matrix metalloproteinase-9 expression in human macrophagic cells through a Toll-like receptor 1 (TLR1). Infect Immun 73:7064–7068
Elkington PT, Nuttall RK, Boyle JJ, O’Kane CM, Horncastle DE et al (2005) Mycobacterium tuberculosis, but not vaccine BCG, specifically upregulates matrix metalloproteinase-1. Am J Respir Crit Care Med 172:1596–1604
Subbian S, Tsenova L, O’Brien P, Yang G, Koo MS et al (2011) Phosphodiesterase-4 inhibition combined with isoniazid treatment of rabbits with pulmonary tuberculosis reduces macrophage activation and lung pathology. Am J Pathol 179:289–301
Elkington P, Shiomi T, Breen R, Nuttall RK, Ugarte-Gil CA et al (2011) MMP-1 drives immunopathology in human tuberculosis and transgenic mice. J Clin Invest 121:1827–1833
Sundararajan S, Babu S, Das SD (2012) Comparison of localized versus systemic levels of matrix metalloproteinases (MMPs), its tissue inhibitors (TIMPs) and cytokines in tuberculous and non-tuberculous pleuritis patients. Hum Immunol 73:985–991
Elkington PT, Green JA, Emerson JE, Lopez-Pascua LD, Boyle JJ et al (2007) Synergistic up-regulation of epithelial cell matrix metalloproteinase-9 secretion in tuberculosis. Am J Respir Cell Mol Biol 37:431–437
Lopez B, Aguilar D, Orozco H, Burger M, Espitia C et al (2003) A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 133:30–37
Manca C, Tsenova L, Bergtold A, Freeman S, Tovey M et al (2001) Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-a/B. Proc Natl Acad Sci USA 98:5752–5757
Manabe YC, Dannenberg AM Jr, Tyagi SK, Hatem CL, Yoder M et al (2003) Different strains of Mycobacterium tuberculosis cause various spectrums of disease in the rabbit model of tuberculosis. Infect Immun 71:6004–6011
Manca C, Reed MB, Freeman S, Mathema B, Kreiswirth B et al (2004) Differential monocyte activation underlies strain-specific Mycobacterium tuberculosis pathogenesis. Infect Immun 72:5511–5514
Gagneux S, Small PM (2007) Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7:328–337
Mathema B, Kurepina NE, Bifani PJ, Kreiswirth BN (2006) Molecular epidemiology of tuberculosis: current insights. Clin Microbiol Rev 19:658–685
Comas I, Chakravartti J, Small PM, Galagan J, Niemann S et al (2010) Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet 42:498–503
Niemann S, Koser CU, Gagneux S, Plinke C, Homolka S et al (2009) Genomic diversity among drug sensitive and multidrug resistant isolates of Mycobacterium tuberculosis with identical DNA fingerprints. PLoS One 4:e7407
Homolka S, Niemann S, Russell DG, Rohde KH (2010) Functional genetic diversity among Mycobacterium tuberculosis complex clinical isolates: delineation of conserved core and lineage-specific transcriptomes during intracellular survival. PLoS Pathog 6:e1000988
Achtman M, Zurth K, Morelli G, Torrea G, Guiyoule A et al (1999) Yersinia pestis, the cause of plague, is a recently emerged clone of Yersinia pseudotuberculosis. Proc Natl Acad Sci U S A 96:14043–14048
Hirsh AE, Tsolaki AG, Deriemer K, Feldman MW, Small PM (2004) Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc Natl Acad Sci U S A 101:4871–4876
Levin BR, Lipsitch M, Bonhoeffer S (1999) Population biology, evolution, and infectious disease: convergence and synthesis. Science 283:806–809
Musser JM (1996) Molecular population genetic analysis of emerged bacterial pathogens: selected insights. Emerg Infect Dis 2:1–17
Friedman CR, Quinn GC, Kreiswirth BN, Perlman DC, Salomon N et al (1997) Widespread dissemination of a drug-susceptible strain of Mycobacterium tuberculosis. J Infect Dis 176:478–484
Soto CY, Menendez MC, Perez E, Samper S, Gomez AB et al (2004) IS6110 mediates increased transcription of the phoP virulence gene in a multidrug-resistant clinical isolate responsible for tuberculosis outbreaks. J Clin Microbiol 42:212–219
Valway SE, Sanchez MPC, Shinnick TF, Orme I, Agerton T et al (1998) An outbreak involving extensive transmission of a virulent strain of Mycobacerium tuberculosis. N Engl J Med 338:633–639
Zhang M, Gong J, Yang Z, Samten B, Cave MD et al (1999) Enhanced capacity of a widespread strain of Mycobacterium tuberculosis to grow in human macrophages. J Infect Dis 179:1213–1217
Reed MB, Domenech P, Manca C, Su H, Barczak AK et al (2004) A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431:84–87
Manca C, Tsenova L, Barry CE III, Bergtold A, Freeman S et al (1999) Mycobacterium tuberculosis CDC1551 induces a more vigorous host response in vivo and in vitro, but is not more virulent than other clinical isolates. J Immunol 162:6740–6746
Newton SM, Smith RJ, Wilkinson KA, Nicol MP, Garton NJ et al (2006) A deletion defining a common Asian lineage of Mycobacterium tuberculosis associates with immune subversion. Proc Natl Acad Sci U S A 103:15594–15598
Portevin D, Gagneux S, Comas I, Young D (2011) Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog 7:e1001307
Chacon-Salinas R, Serafin-Lopez J, Ramos-Payan R, Mendez-Aragon P, Hernandez-Pando R et al (2005) Differential pattern of cytokine expression by macrophages infected in vitro with different Mycobacterium tuberculosis genotypes. Clin Exp Immunol 140:443–449
Dormans J, Burger M, Aguilar D, Hernandez-Pando R, Kremer K et al (2004) Correlation of virulence, lung pathology, bacterial load and delayed type hypersensitivity responses after infection with different Mycobacterium tuberculosis genotypes in a BALB/c mouse model. Clin Exp Immunol 137:460–468
Shimono N, Morici L, Casali N, Cantrell S, Sidders B et al (2003) Hypervirulent mutant of Mycobacterium tuberculosis resulting from disruption of the mce1 operon. Proc Natl Acad Sci U S A 100:15918–15923
Subbian S, Tsenova L, Yang G, O’Brien P, Parsons S et al (2011) Chronic pulmonary cavitary tuberculosis in rabbits: a failed host immune response. Open Biol 1:110016
Subbian S, Tsenova L, O’Brien P, Yang G, Kushner NL et al (2012) Spontaneous latency in a rabbit model of pulmonary tuberculosis. Am J Pathol 181:1711–1724
Subbian S, O’Brien P, Kushner NL, Yang G, Tsenova L et al (2013) Molecular immunologic correlates of spontaneous latency in a rabbit model of pulmonary tuberculosis. Cell Commun Signal 11:16
Briken V, Porcelli SA, Besra GS, Kremer L (2004) Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol 53:391–403
Torrelles JB, Schlesinger LS (2010) Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis 90:84–93
Crick DC, Brennan PJ, McNeil MR (2003) The cell wall of Mycobacterium tuberculosis. In: Rom WM, Garay SM (eds) Tuberculosis, 2nd edn. Lippincott Williams and Wilkins, Philadelphia
Rajaram MV, Ni B, Morris JD, Brooks MN, Carlson TK et al (2011) Mycobacterium tuberculosis lipomannan blocks TNF biosynthesis by regulating macrophage MAPK-activated protein kinase 2 (MK2) and microRNA miR-125b. Proc Natl Acad Sci U S A 108:17408–17413
Torrelles JB, Sieling PA, Arcos J, Knaup R, Bartling C et al (2011) Structural differences in lipomannans from pathogenic and nonpathogenic mycobacteria that impact CD1b-restricted T cell responses. J Biol Chem 286:35438–35446
Yadav M, Roach SK, Schorey JS (2004) Increased mitogen-activated protein kinase activity and TNF-alpha production associated with Mycobacterium smegmatis- but not Mycobacterium avium-infected macrophages requires prolonged stimulation of the calmodulin/calmodulin kinase and cyclic AMP/protein kinase A pathways. J Immunol 172:5588–5597
Doz E, Rose S, Nigou J, Gilleron M, Puzo G et al (2007) Acylation determines the toll-like receptor (TLR)-dependent positive versus TLR2-, mannose receptor-, and SIGNR1-independent negative regulation of pro-inflammatory cytokines by mycobacterial lipomannan. J Biol Chem 282:26014–26025
Guerardel Y, Maes E, Elass E, Leroy Y, Timmerman P et al (2002) Structural study of lipomannan and lipoarabinomannan from Mycobacterium chelonae. Presence of unusual components with alpha 1,3-mannopyranose side chains. J Biol Chem 277:30635–30648
Hance AJ, Saltini C, Crystal RG (1988) Does de novo immunoglobin synthesis occur on the epithelial surface of the human lower respiratory tract? Am Rev Resp Dis 137:17–24
Khoo K-H, Dell A, Morris HR, Brennan PJ, Chatterjee D (1995) Inositol phosphate capping of the nonreducing termini of lipoarabinomannan from rapidly growing strains of Mycobacterium. J Biol Chem 270:12380–12389
Afonso-Barroso A, Clark SO, Williams A, Rosa GT, Nobrega C et al (2012) Lipoarabinomannan mannose caps do not affect mycobacterial virulence or the induction of protective immunity in experimental animal models of infection and have minimal impact on in vitro inflammatory responses. Cell Microbiol. doi:10.1111/cmi.12065
Author information
Authors and Affiliations
Corresponding authors
Additional information
This article is a contribution to the special issue on Macrophage Heterogeneity, Subsets and Human Disease - Guest Editor: Siamon Gordon
Rights and permissions
About this article
Cite this article
Guirado, E., Schlesinger, L.S. & Kaplan, G. Macrophages in tuberculosis: friend or foe. Semin Immunopathol 35, 563–583 (2013). https://doi.org/10.1007/s00281-013-0388-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00281-013-0388-2