Abstract.
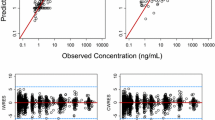
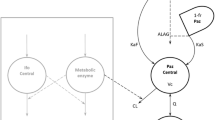
Purpose: KRN5500, a novel spicamycin derivative, shows the greatest activity against a human tumor xenograft model and the highest therapeutic index among spicamycin derivatives. KRN5500 is currently under clinical development in Japan and the United States. The objective of this study was to develop a population pharmacokinetic model that describes the KRN5500 plasma concentration versus time data. Methods: Data were collected from 18 patients entered in a phase 1 study. These patients received KRN5500 3–21 mg/m2 as a 2-h infusion. A total of 219 concentration measurements were available. The data were analyzed using the nonlinear mixed effect model (NONMEM) program. In addition, the basic and final population pharmacokinetic models were evaluated using bootstrapping resampling. Results: The basic model selected was a two-compartment model with a combination of additive and constant coefficient of variation error models. The basic model fitted well not only the original data, but also 100 bootstrap replicates generated from the original data set. With regard to the effect of covariates selected by generalized additive modeling analysis, gender (SEX) and performance status were found to be possible determinants of the volume of central compartment by NONMEM analysis. The final regression model for V1 was V1=θ<SUB>V1</SUB>(1-SEX×θ<SUB>SEX</SUB>), where V1 is the typical population value of the volume of central compartment, and SEX=0 if the patient is male, otherwise SEX=1. The final model was fitted to the 200 bootstrapped samples. The mean parameter estimates were within 15% of those obtained with the original data set. Conclusions: The KRN5500 plasma concentration versus time data obtained from the phase 1 study were well described by the population pharmacokinetic model. Further evaluation by bootstrapping showed that the population pharmacokinetic model was stable.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Takama, H., Tanaka, H., Sudo, T. et al. Population pharmacokinetic modeling and model validation of a spicamycin derivative, KRN5500, in phase 1 study. Cancer Chemother Pharmacol 47, 404–410 (2001). https://doi.org/10.1007/s002800000257
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002800000257