Abstract
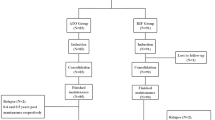
Realgar-Indigo naturalis formula (RIF) is a traditional Chinese medicine containing As4S4 and effective in treating acute promyelocytic leukemia (APL). The dose of RIF remains to be determined in pediatric patients. Comparison of plasma arsenic concentrations and toxicity between RIF and arsenic trioxide (ATO) treatment in APL may help to establish an appropriate therapeutic dose of RIF for children. From October 2018 to March 2020, 19 pediatric patients with APL treated with SCCLG-APL protocol were included, 9 in RIF group at 135 mg/kg/day orally three times daily, and 10 in ATO group at 0.16 mg/kg/day intravenously over 12 h daily. Peak and trough plasma arsenic concentrations were assayed at D1, 2, 7 and 14 of induction treatment. Urine arsenic excretions were assessed with spot urine samples and the measurements were adjusted using creatinine. Toxicities were compared between two groups. The plasma arsenic concentration reached steady state at D7 either in the RIF or ATO group, and the mean peak and trough concentrations were similar between two groups (P > 0.05), which were 0.54 μmol/L and 0.48 μmol/L in RIF group, and 0.63 μmol/L and 0.51 μmol/L in ATO group, respectively. Urine arsenic excretion rate was positively correlated with the concentration of plasma arsenic. The rates of treatment-related adverse events were similar in two groups. In conclusion, the dose of RIF at 135 mg/kg/day may be an appropriate therapeutic dose in children with APL. Urine arsenic level can be used as an indicator to estimate plasma arsenic concentration. Trial registration www.clinicaltrials.gov NCT02200978.



Similar content being viewed by others
References
Yang MH, Wan WQ, Luo JS et al (2018) Multicenter randomized trial of arsenic trioxide and Realgar-Indigo naturalis formula in pediatric patients with acute promyelocytic leukemia: Interim results of the SCCLG-APL clinical study. Am J Hematol 93(12):1467–1473. https://doi.org/10.1002/ajh.25271
Zhu HH, Wu DP, Jin J et al (2013) Oral tetra-arsenic tetra-sulfide formula versus intravenous arsenic trioxide as first-line treatment of acute promyelocytic leukemia: a multicenter randomized controlled trial. J Clin Oncol 31:4215–4221. https://doi.org/10.1200/JCO.2013.48.8312
Wang L, Zhou GB, Liu P et al (2008) Dissection of mechanisms of Chinese medicinal formula Realgar-indigo naturalis as an effective treatment for promyelocytic leukemia. Proc Natl Acad Sci USA 105:4826–4831. https://doi.org/10.1073/pnas.0712365105
Bi LL, Ma Q, Wang SQ et al (2005) Treatment of 32 cases with recurring acute promyelocytic leukemia with tablets of composite natural indigo. Zhonghua Er Ke Za Zhi Sep 43(9):702–703. https://doi.org/10.3760/j.issn:0578-1310.2005.09.020
Luo XQ, Ke ZY, Huang LB et al (2009) Improved outcome for Chinese children with acute promyelocytic leukemia: a comparison of two protocols. Pediatr Blood Cancer 53(3):325–328. https://doi.org/10.1002/pbc.22042
Wang J, Huang JB, Liu ZL et al (2017) Comparison of curative effect between Fu Fang Huang Dai Pian and Arsenic Trioxide in treatment of 45 patients with acute promyelocytic leukaemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi 25:1605–1610. https://doi.org/10.7534/j.issn.1009-2137.2017.06.004
Pediatric Hematology and Oncology Committee, Chinese Medical Doctor Association Pediatrician Branch; Subspecialty Group of Hematology, the Society of Pediatrics, Chinese Medical Association (2019) Interpretation of the Chinese guideline for diagnosis and treatment of childhood acute promyelocytic leukemia (2018). Zhonghua Er Ke Za Zhi 57(10):757–760. https://doi.org/10.3760/cma.j.issn.0578-1310.2019.10.006
Chen GQ, Shi XG, Tang W et al (1997) Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood 89:3345–3353. https://doi.org/10.1182/blood.V89.9.3345
Guo M, Zhou J, Fan S et al (2021) Characteristics and clinical influence factors of arsenic species in plasma and their role of arsenic species as predictors for clinical efficacy in acute promyelocytic leukemia (APL) patients treated with arsenic trioxide. Expert Rev Clin Pharmacol 14(4):503–512. https://doi.org/10.1080/17512433.2021.1893940
Meihua G, Jing Li, Shengjin F et al (2019) Speciation analysis of arsenic in urine samples from APL patients treated with single agent As2O3 by HPLC-HG-AFS. J Pharm Biomed Anal 171:212–217. https://doi.org/10.1016/j.jpba.2019.04.014
Fang Wang F, Jia JS, Wang J et al (2017) The kinetics of white blood cell and the predictive factors of leukocytosis under oral or intravenous arsenic as the first-line treatment for acute promyelocytic leukemia. Leukemia Res 61:84–88. https://doi.org/10.1016/j.leukres.2017.09.006
Zheng H, Jiang H, Hu S et al (2021) Arsenic combined with all-trans retinoic acid for pediatric acute promyelocytic leukemia: report from the CCLG-APL2016 protocol study. J Clin Oncol 39(28):3161–3170. https://doi.org/10.1200/JCO.20.03096
Zhang L, Yang XM, Chen J et al (2021) Population pharmacokinetics and safety of oral tetra-arsenic tetra-sulfide formula in pediatric acute promyelocytic leukemia. Drug Des Dev Ther 15:1633–1640. https://doi.org/10.2147/DDDT.S305244
Fox E, Razzouk BI, Widemann BC et al (2008) Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood 111(2):566–573. Doi: https://doi.org/10.1182/blood-2007-08-107839
Xiang Y, Wang XB, Sun SJ et al (2009) Compound huangdai tablet as induction therapy for 193 patients with acute promyelocytic leukemia. Zhonghua Xue Ye Xue Za Zhi 30(7):440–442. https://doi.org/10.3760/cma.j.issn.0253-2727.2009.07.004
Wang FR, Lou YQ, Lu DP (2005) A clinical pharmacokinetic study of multi-dose oral tetra-arsenic tetra-sulfide combination therapy in acute promyelocytic leukemia. Zhonghua Nei Ke Za Zhi 44(10):15–18. https://doi.org/10.3760/j.issn:0578-1426.2005.10.005
Howe CG, Liu H, Hall MN et al (2016) Associations between blood and urine arsenic concentrations and global levels of post-translational histone modifications in bangladeshi men and women. Environ Health Perspect 124(8):1234–1240. https://doi.org/10.1289/ehp.1510412
Zhou J, Meng R, Sui X et al (2005) Effects of administration styles of arsenic trioxide on intracellular arsenic concentration, cell differentiation and apoptosis. Haematologica 90(9):1277 (PMID: 16154855)
Gao C, Hu S, Guo M et al (2018) Clinical pharmacokinetics and safety profile of single agent arsenic trioxide by continuous slow-rate infusion in patients with newly diagnosed acute promyelocytic leukemia. Cancer Chemother Pharmacol 82(2):229–236. https://doi.org/10.1007/s00280-018-3606-8
Liu J, Lu Y, Wu Q et al (2008) Mineral arsenicals in traditional medicines: orpiment, realgar, and arsenolite. J Pharmacol Exp Ther 326(2):363–368. https://doi.org/10.1124/jpet.108.139543
Acknowledgements
We thank the patients and their families for th eir willingness to participate in this trial, and all participants and research staff of all centers within SCCLG.
Funding
The Science and Technology Planning Project of Guangdong Province, China (Grant/Award Number: 2014A020221008), the Science and Technology Planning Project of Guangzhou, China (Grant/Award Number: 20160402012) and the Scientific research project of Guangdong Provincial Bureau of Traditional Chinese Medicine, China (Grant/Award Number: 20201056).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, LH., Chen, YQ., Huang, DP. et al. The comparison of plasma arsenic concentration and urinary arsenic excretion during treatment with Realgar-Indigo naturalis formula and arsenic trioxide in children with acute promyelocytic leukemia. Cancer Chemother Pharmacol 90, 45–52 (2022). https://doi.org/10.1007/s00280-022-04449-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-022-04449-9