Abstract
Purpose
Polyamines are absolutely essential for maintaining tumor cell proliferation. PG-11047, a polyamine analogue, is a nonfunctional competitor of the natural polyamine spermine that has demonstrated anticancer activity in cells and animal models of multiple cancer types. Preclinical investigations into the effects of common chemotherapeutic agents have revealed overlap with components of the polyamine metabolic pathway also affected by PG-11047. This report describes a Phase Ib clinical trial investigating PG-11047 in combination with cytotoxic and anti-angiogenic chemotherapeutic agents in patients with advanced refractory metastatic solid tumors or lymphoma.
Methods
A total of 172 patients were assigned to treatment arms based on cancer type to receive the appropriate standard-of-care therapy (gemcitabine, docetaxel, bevacizumab, erlotinib, cisplatin, 5-fluorouracil (5-FU), or sunitinib as directed) along with once weekly intravenous infusions of PG-11047. PG-11047 dose escalation ranged from 50 to 590 mg.
Results
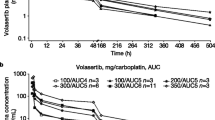
The maximum tolerated dose (MTD) of PG-11047 in combination with bevacizumab, erlotinib, cisplatin, and 5-FU was 590 mg. Dose-limiting toxicities (DLTs) in these groups were rare (5 of 148 patients). Overall partial responses (PR) were observed in 12% of patients treated with PG-11047 and bevacizumab, with stable disease documented in an additional 40%. Stable disease occurred in 71.4% of patients in the 5-FU arm, 54.1% in the cisplatin arm, and 33.3% in the erlotinib arm. Four of the patients receiving cisplatin + PG-11047 (20%) had unconfirmed PRs. MTDs for gemcitabine, docetaxel, and sunitinib could not be determined due to DLTs at low doses of PG-11047 and small sample size.
Conclusions
Results of this Phase Ib trial indicate that PG-11047 can be safely administered to patients in combination with bevacizumab, erlotinib, cisplatin, and 5-FU on the once weekly dosing schedule described and may provide therapeutic benefit. The manageable toxicity profile and high MTD determination provide a safety profile for further clinical studies.
Similar content being viewed by others
Data availability
Data collected during the study are available on www.clinicaltrials.gov, #NCT00705874.
References
Flynn AT, Hogarty MD (2018) Myc, oncogenic protein translation, and the role of polyamines. Med Sci (Basel) 6:2. https://doi.org/10.3390/medsci6020041
Casero RA Jr, Marton LJ (2007) Targeting polyamine metabolism and function in cancer and other hyperproliferative diseases. Nat Rev Drug Discov 6(5):373–390. https://doi.org/10.1038/nrd2243
Murray-Stewart TR, Woster PM, Casero RA Jr (2016) Targeting polyamine metabolism for cancer therapy and prevention. Biochem J 473(19):2937–2953. https://doi.org/10.1042/BCJ20160383
Casero RA Jr, Murray Stewart T, Pegg AE (2018) Polyamine metabolism and cancer: treatments, challenges and opportunities. Nat Rev Cancer 18(11):681–695. https://doi.org/10.1038/s41568-018-0050-3
Raj KP, Zell JA, Rock CL, McLaren CE, Zoumas-Morse C, Gerner EW, Meyskens FL (2013) Role of dietary polyamines in a phase III clinical trial of difluoromethylornithine (DFMO) and sulindac for prevention of sporadic colorectal adenomas. Br J Cancer 108(3):512–518. https://doi.org/10.1038/bjc.2013.15
Meyskens FL Jr, Gerner EW (1999) Development of difluoromethylornithine (DFMO) as a chemoprevention agent. Clin Cancer Res 5(5):945–951
Saulnier Sholler GL, Gerner EW, Bergendahl G, MacArthur RB, VanderWerff A, Ashikaga T, Bond JP, Ferguson W, Roberts W, Wada RK, Eslin D, Kraveka JM, Kaplan J, Mitchell D, Parikh NS, Neville K, Sender L, Higgins T, Kawakita M, Hiramatsu K, Moriya SS, Bachmann AS (2015) A phase I trial of DFMO targeting polyamine addiction in patients with relapsed/refractory neuroblastoma. PLoS ONE 10(5):e0127246. https://doi.org/10.1371/journal.pone.0127246
Lewis EC, Kraveka JM, Ferguson W, Eslin D, Brown VI, Bergendahl G, Roberts W, Wada RK, Oesterheld J, Mitchell D, Foley J, Zage P, Rawwas J, Rich M, Lorenzi E, Broglio K, Berry D, Saulnier Sholler GL (2020) A subset analysis of a phase II trial evaluating the use of DFMO as maintenance therapy for high-risk neuroblastoma. Int J Cancer 147(11):3152–3159. https://doi.org/10.1002/ijc.33044
Sholler GLS, Ferguson W, Bergendahl G, Bond JP, Neville K, Eslin D, Brown V, Roberts W, Wada RK, Oesterheld J, Mitchell D, Foley J, Parikh NS, Eshun F, Zage P, Rawwas J, Sencer S, Pankiewicz D, Quinn M, Rich M, Junewick J, Kraveka JM (2018) Maintenance DFMO increases survival in high risk neuroblastoma. Sci Rep 8(1):14445. https://doi.org/10.1038/s41598-018-32659-w
Meyskens FL, Simoneau AR, Gerner EW (2014) Chemoprevention of prostate cancer with the polyamine synthesis inhibitor difluoromethylornithine. Recent Results Cancer Res 202:115–120. https://doi.org/10.1007/978-3-642-45195-9_14
Levin VA, Ictech SE, Hess KR (2018) Clinical importance of eflornithine (α-difluoromethylornithine) for the treatment of malignant gliomas. CNS Oncol 7(2):CNS16. https://doi.org/10.2217/cns-2017-0031
Battaglia V, DeStefano SC, Murray-Stewart T, Casero RA Jr (2014) Polyamine catabolism in carcinogenesis: potential targets for chemotherapy and chemoprevention. Amino Acids 46(3):511–519. https://doi.org/10.1007/s00726-013-1529-6
Casero RA Jr, Woster PM (2001) Terminally alkylated polyamine analogues as chemotherapeutic agents. J Med Chem 44(1):1–26
Libby PR, Bergeron RJ, Porter CW (1989) Structure-function correlations of polyamine analog-induced increases in spermidine/spermine acetyltransferase activity. Biochem Pharmacol 38(9):1435–1442 (0006-2952(89)90182-2[pii])
Casero RA Jr, Celano P, Ervin SJ, Porter CW, Bergeron RJ, Libby PR (1989) Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res 49(14):3829–3833
Porter CW, McManis J, Casero RA, Bergeron RJ (1987) Relative abilities of bis(ethyl) derivatives of putrescine, spermidine, and spermine to regulate polyamine biosynthesis and inhibit L1210 leukemia cell growth. Cancer Res 47(11):2821–2825
Devereux W, Wang Y, Stewart TM, Hacker A, Smith R, Frydman B, Valasinas AL, Reddy VK, Marton LJ, Ward TD, Woster PM, Casero RA (2003) Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother Pharmacol 52(5):383–390
Ha HC, Sirisoma NS, Kuppusamy P, Zweier JL, Woster PM, Casero RA Jr (1998) The natural polyamine spermine functions directly as a free radical scavenger. Proc Natl Acad Sci USA 95(19):11140–11145
Murray Stewart T, Dunston TT, Woster PM, Casero RA Jr (2018) Polyamine catabolism and oxidative damage. J Biol Chem 293(48):18736–18745. https://doi.org/10.1074/jbc.TM118.003337
Reddy VK, Valasinas A, Sarkar A, Basu HS, Marton LJ, Frydman B (1998) Conformationally restricted analogues of 1N,12N-bisethylspermine: synthesis and growth inhibitory effects on human tumor cell lines. J Med Chem 41(24):4723–4732. https://doi.org/10.1021/jm980172v
Hahm HA, Ettinger DS, Bowling K, Hoker B, Chen TL, Zabelina Y, Casero RA Jr (2002) Phase I study of N(1), N(11)-diethylnorspermine in patients with non-small cell lung cancer. Clin Cancer Res 8(3):684–690
Creaven PJ, Perez R, Pendyala L, Meropol NJ, Loewen G, Levine E, Berghorn E, Raghavan D (1997) Unusual central nervous system toxicity in a phase I study of N1N11 diethylnorspermine in patients with advanced malignancy. Invest New Drugs 15(3):227–234
Hacker A, Marton LJ, Sobolewski M, Casero RA Jr (2008) In vitro and in vivo effects of the conformationally restricted polyamine analogue CGC-11047 on small cell and non-small cell lung cancer cells. Cancer Chemother Pharmacol 63(1):45–53
Dredge K, Kink JA, Johnson RM, Bytheway I, Marton LJ (2009) The polyamine analog PG11047 potentiates the antitumor activity of cisplatin and bevacizumab in preclinical models of lung and prostate cancer. Cancer Chemother Pharmacol 65(1):191–195. https://doi.org/10.1007/s00280-009-1105-7
Holst CM, Frydman B, Marton LJ, Oredsson SM (2006) Differential polyamine analogue effects in four human breast cancer cell lines. Toxicology 223(1–2):71–81. https://doi.org/10.1016/j.tox.2006.03.009 (S0300-483X(06)00155-7[pii])
Kuo WL, Das D, Ziyad S, Bhattacharya S, Gibb WJ, Heiser LM, Sadanandam A, Fontenay GV, Hu Z, Wang NJ, Bayani N, Feiler HS, Neve RM, Wyrobek AJ, Spellman PT, Marton LJ, Gray JW (2009) A systems analysis of the chemosensitivity of breast cancer cells to the polyamine analogue PG-11047. BMC Med 7:77. https://doi.org/10.1186/1741-7015-7-77 (1741-7015-7-77[pii])
Ignatenko NA, Yerushalmi HF, Pandey R, Kachel KL, Stringer DE, Marton LJ, Gerner EW (2009) Gene expression analysis of HCT116 colon tumor-derived cells treated with the polyamine analog PG-11047. Cancer Genom Proteom 6(3):161–175 (6/3/161[pii])
Smith MA, Maris JM, Lock R, Kolb EA, Gorlick R, Keir ST, Carol H, Morton CL, Reynolds CP, Kang MH, Houghton PJ (2011) Initial testing (stage 1) of the polyamine analog PG11047 by the pediatric preclinical testing program. Pediatr Blood Cancer 57(2):268–274. https://doi.org/10.1002/pbc.22797
Cirenajwis H, Smiljanic S, Honeth G, Hegardt C, Marton LJ, Oredsson SM (2010) Reduction of the putative CD44+CD24- breast cancer stem cell population by targeting the polyamine metabolic pathway with PG11047. Anticancer Drugs 21(10):897–906. https://doi.org/10.1097/CAD.0b013e32833f2f77
Murray Stewart T, Desai AA, Fitzgerald ML, Marton LJ, Casero RA Jr (2020) A phase I dose-escalation study of the polyamine analog PG-11047 in patients with advanced solid tumors. Cancer Chemother Pharmacol 85(6):1089–1096. https://doi.org/10.1007/s00280-020-04082-4
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the united states, national cancer institute of Canada. J Nat Cancer Instit 92(3):205–216. https://doi.org/10.1093/jnci/92.3.205
Pouessel D, Culine S (2008) High frequency of intracerebral hemorrhage in metastatic renal carcinoma patients with brain metastases treated with tyrosine kinase inhibitors targeting the vascular endothelial growth factor receptor. Eur Urol 53(2):376–381. https://doi.org/10.1016/j.eururo.2007.08.053
Hager S, Ackermann CJ, Joerger M, Gillessen S, Omlin A (2016) Anti-tumour activity of platinum compounds in advanced prostate cancer-a systematic literature review. Ann Oncol 27(6):975–984. https://doi.org/10.1093/annonc/mdw156
Goldfarb SB, Hudis C, Dickler MN (2011) Bevacizumab in metastatic breast cancer: when may it be used? Ther Adv Med Oncol 3(2):85–93. https://doi.org/10.1177/1758834010397627
Svensson KJ, Welch JE, Kucharzewska P, Bengtson P, Bjurberg M, Pahlman S, Ten Dam GB, Persson L, Belting M (2008) Hypoxia-mediated induction of the polyamine system provides opportunities for tumor growth inhibition by combined targeting of vascular endothelial growth factor and ornithine decarboxylase. Cancer Res 68(22):9291–9301. https://doi.org/10.1158/0008-5472.CAN-08-2340
Varma R, Hector S, Greco WR, Clark K, Hawthorn L, Porter C, Pendyala L (2007) Platinum drug effects on the expression of genes in the polyamine pathway: time-course and concentration-effect analysis based on Affymetrix gene expression profiling of A2780 ovarian carcinoma cells. Cancer Chemother Pharmacol 59(6):711–723. https://doi.org/10.1007/s00280-006-0325-3
Allen WL, McLean EG, Boyer J, McCulla A, Wilson PM, Coyle V, Longley DB, Casero RA Jr, Johnston PG (2007) The role of spermidine/spermine N1-acetyltransferase in determining response to chemotherapeutic agents in colorectal cancer cells. Mol Cancer Ther 6(1):128–137. https://doi.org/10.1158/1535-7163.MCT-06-0303 (6/1/128[pii])
Funding
Funding for portions of this research was provided by the National Institutes of Health National Cancer Institute (R01CA204345 and R01CA235863 to RAC).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
During the development of PG-11047, in vitro and in vivo preclinical studies of PG-11047 and related analogues were funded in part by a gift to the laboratory of RAC by Cellgate, Inc., the previous owner of PG-11047.
Ethics approval and consent to participate
All participants provided written informed consent that was approved by the Institutional Review Boards (IRBs) associated with the study site prior to study initiation. The trial was conducted in accordance with the IRB-approved protocol and amendments, Good Clinical Practice guidelines, and the Declaration of Helsinki.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Murray Stewart, T., Von Hoff, D., Fitzgerald, M. et al. A Phase Ib multicenter, dose-escalation study of the polyamine analogue PG-11047 in combination with gemcitabine, docetaxel, bevacizumab, erlotinib, cisplatin, 5-fluorouracil, or sunitinib in patients with advanced solid tumors or lymphoma. Cancer Chemother Pharmacol 87, 135–144 (2021). https://doi.org/10.1007/s00280-020-04201-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-020-04201-1