Abstract
Purpose
To evaluate the effect of lapatinib on the QTc interval and ECG parameters in patients with advanced solid tumors.
Methods
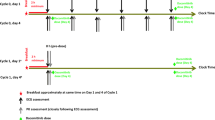
This was a multicenter, placebo-controlled study in subjects with advanced solid tumors. Subjects were administered two doses of matching placebo on day 1, 12 h apart and one dose in the morning on day 2. Two doses of lapatinib 2000 mg were administered orally on day 3, 12 h apart and one dose in the morning on day 4. Twelve-lead digital ECGs were extracted from continuous Holter recordings at pre-specified time points over the 24-h period on days 2 and 4. Venous blood samples for lapatinib concentrations were obtained immediately following the ECGs.
Results
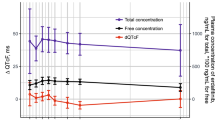
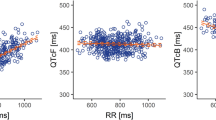
A maximum mean baseline-adjusted, placebo time-matched increase in QTcF, (ddQTcF) in the evaluable, (EV) population (n = 37) of 8.8 ms (90% CI 4.1, 13.4) occurred approximately 10 h after the third lapatinib dose. These results were consistent with those in the pharmacodynamic, PD population, (n = 52) (ddQTcF = 7.9 ms; 90% CI 4.1, 11.7). No subject experienced QTcF increases from baseline of > 60 ms on lapatinib or placebo. The geometric mean lapatinib Cmax of 3902 ng/mL was observed at 3.6 h post-dose.
Conclusions
These data show a relevant, treatment-related increase in QTcF after treatment with three doses of lapatinib 2000 mg. This study confirms the need for caution in patients with solid tumors treated with lapatinib, and who are concomitantly receiving drugs that are strong CYP3A inhibitors and/or prolong the QTc.




Similar content being viewed by others
References
Reid A, Vidal L, Shaw H, de Bono J (2007) Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu). Eur J Cancer 43:481–489. https://doi.org/10.1016/j.ejca.2006.11.007
Xia W, Mullin RJ, Keith BR, Liu LH, Ma H, Rusnak DW, Owens G, Alligood KJ, Spector NL (2002) Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 21:6255–6263. https://doi.org/10.1038/sj.onc.1205794
Tykerb(R) [package insert]. Novartis Pharmaceutical Corporation (2017). https://www.pharma.us.novartis.com/sites/www.pharma.us.novartis.com/files/tykerb.pdf. Accessed Mar 2018
Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355:2733–2743. https://doi.org/10.1056/NEJMoa064320
Kloth JS, Pagani A, Verboom MC, Malovini A, Napolitano C, Kruit WH, Sleijfer S, Steeghs N, Zambelli A, Mathijssen RH (2015) Incidence and relevance of QTc-interval prolongation caused by tyrosine kinase inhibitors. Br J Cancer 112:1011–1016. https://doi.org/10.1038/bjc.2015.82
Miles DR, Lacy SA, Wada DR, Milwee S, Yaron Y, Nguyen LT (2017) Assessment of cabozantinib treatment on QT interval in a phase 3 study in medullary thyroid cancer: evaluation of indirect QT effects mediated through treatment-induced changes in serum electrolytes. Cancer Chemother Pharmacol 80:295–306. https://doi.org/10.1007/s00280-017-3349-y
Shah RR, Morganroth J (2015) Update on cardiovascular safety of tyrosine kinase inhibitors: with a special focus on QT interval, left ventricular dysfunction and overall risk/benefit. Drug Saf 38:693–710. https://doi.org/10.1007/s40264-015-0300-1
Shah RR, Morganroth J, Shah DR (2013) Cardiovascular safety of tyrosine kinase inhibitors: with a special focus on cardiac repolarisation (QT interval). Drug Saf 36:295–316. https://doi.org/10.1007/s40264-013-0047-5
Dong Q, Fu XX, Du LL, Zhao N, Xia CK, Yu KW, Cheng LX, Du YM (2013) Blocking of the human ether-a-go-go-related gene channel by imatinib mesylate. Biol Pharm Bull 36:268–275
Strevel EL, Ing DJ, Siu LL (2007) Molecularly targeted oncology therapeutics and prolongation of the QT interval. J Clin Oncol 25:3362–3371. https://doi.org/10.1200/jco.2006.09.6925
Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, Lin RZ (2012) Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med 4:131ra150. https://doi.org/10.1126/scitranslmed.3003623
Agency EM (2008) Assessment report for Tyverb. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/000795/WC500044960.pdf. Accessed 19 Jan 2019
Lee HA, Kim EJ, Hyun SA, Park SG, Kim KS (2010) Electrophysiological effects of the anti-cancer drug lapatinib on cardiac repolarization. Basic Clin Pharmacol Toxicol 107:614–618. https://doi.org/10.1111/j.1742-7843.2010.00556.x
Koch KM, Reddy NJ, Cohen RB, Lewis NL, Whitehead B, Mackay K, Stead A, Beelen AP, Lewis LD (2009) Effects of food on the relative bioavailability of lapatinib in cancer patients. J Clin Oncol 27:1191–1196. https://doi.org/10.1200/jco.2008.18.3285
Dogan E, Yorgun H, Petekkaya I, Ozer N, Altundag K, Ozisik Y (2012) Evaluation of cardiac safety of lapatinib therapy for ErbB2-positive metastatic breast cancer: a single center experience. Med Oncol (Northwood, London, England) 29:3232–3239. https://doi.org/10.1007/s12032-012-0253-5
Chien AJ, Illi JA, Ko AH, Korn WM, Fong L, Chen LM, Kashani-Sabet M, Ryan CJ, Rosenberg JE, Dubey S, Small EJ, Jahan TM, Hylton NM, Yeh BM, Huang Y, Koch KM, Moasser MM (2009) A phase I study of a 2-day lapatinib chemosensitization pulse preceding nanoparticle albumin-bound paclitaxel for advanced solid malignancies. Clin Cancer Res 15:5569–5575. https://doi.org/10.1158/1078-0432.ccr-09-0522
Desai M, Li L, Desta Z, Malik M, Flockhart D (2003) Variability of heart rate correction methods for the QT interval. Br J Clin Pharmacol 55:511–517
Malik M (2001) Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol 12:411–420
Hsieh S, Tobien T, Koch K, Dunn J (2004) Increasing throughput of parallel on-line extraction liquid chromatography/electrospray ionization tandem mass spectrometry system for GLP quantitative bioanalysis in drug development. Rapid Commun Mass Spectrom 18:285–292. https://doi.org/10.1002/rcm.1327
GlaxoSmithKline (2007) A phase I, open-label, multiple dose, dose-escalation study of GW572016 in patients with solid tumors. https://www.gsk-studyregister.com/study/7491. Accessed 19 Jan 2019
Administration UFaD (2005) E14 Clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM073153.pdf. Accessed 21 Aug 2017
Harmonisation ICf (2016) ICH E14 guideline: the clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non-antiarrhythmic drugs questions & answers (R3). http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Q_As_R3__Step4.pdf. Accessed 3 Mar 2018
Al-Khatib SM, LaPointe NM, Kramer JM, Califf RM (2003) What clinicians should know about the QT interval. JAMA 289:2120–2127. https://doi.org/10.1001/jama.289.16.2120
Trinkley KE, Page RL 2nd, Lien H, Yamanouye K, Tisdale JE (2013) QT interval prolongation and the risk of torsades de pointes: essentials for clinicians. Curr Med Res Opin 29:1719–1726. https://doi.org/10.1185/03007995.2013.840568
Hondeghem LM (2018) Drug-induced QT prolongation and torsades de pointes: an all-exclusive relationship or time for an amicable separation? Drug Saf 41:11–17. https://doi.org/10.1007/s40264-017-0584-4
Therapeutics ACfE, Research o (2018) CredibleMeds :: QTDrugs lists (registration required). https://crediblemeds.org/new-drug-list. Accessed 25 Mar 2018
Castellino S, O’Mara M, Koch K, Borts DJ, Bowers GD, MacLauchlin C (2012) Human metabolism of lapatinib, a dual kinase inhibitor: implications for hepatotoxicity. Drug Metab Dispos 40:139–150. https://doi.org/10.1124/dmd.111.040949
Towles JK, Clark RN, Wahlin MD, Uttamsingh V, Rettie AE, Jackson KD (2016) Cytochrome P450 3A4 and CYP3A5-catalyzed bioactivation of lapatinib. Drug Metab Dispos 44:1584–1597. https://doi.org/10.1124/dmd.116.070839
Abbas R, Hug BA, Leister C, Sonnichsen D (2012) A randomized, crossover, placebo- and moxifloxacin-controlled study to evaluate the effects of bosutinib (SKI-606), a dual Src/Abl tyrosine kinase inhibitor, on cardiac repolarization in healthy adult subjects. Int J Cancer 131:E304–E311. https://doi.org/10.1002/ijc.27348
Heath EI, Infante J, Lewis LD, Luu T, Stephenson J, Tan AR, Kasubhai S, LoRusso P, Ma B, Suttle AB, Kleha JF, Ball HA, Dar MM (2013) A randomized, double-blind, placebo-controlled study to evaluate the effect of repeated oral doses of pazopanib on cardiac conduction in patients with solid tumors. Cancer Chemother Pharmacol 71:565–573. https://doi.org/10.1007/s00280-012-2030-8
Kim TD, le Coutre P, Schwarz M, Grille P, Levitin M, Fateh-Moghadam S, Giles FJ, Dorken B, Haverkamp W, Kohncke C (2012) Clinical cardiac safety profile of nilotinib. Haematologica 97:883–889. https://doi.org/10.3324/haematol.2011.058776
Acknowledgements
We thank our patients and their families without whom this study would not have been possible. We also wish to thank our colleague oncologists, research nurses and study coordinators at each study site.
Funding
Original funding for this study (NCT01328054) was provided by GlaxoSmithKline. As of March 2015 lapatinib is an asset of Novartis Pharmaceutical Corporation, the current sponsor of this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
SAC: Has declared no conflicts of interest regarding the conduct of this clinical trial or drafting the manuscript. HIH: Received research funding from GlaxoSmithKline, paid through Duke University Medical Center at the time of this work and drafting the manuscript. He is currently employed by Genentech/Roche and owns Roche stock. SS: Received research funding from GlaxoSmithKline/Novartis at the time of this work and has served on GlaxoSmithKline and Novartis Advisory Boards. DW: Has declared no conflicts of interest regarding the conduct of this clinical trial or drafting the manuscript. JPZ: Is employed by Novartis. LDL: Received research funding from GlaxoSmithKline/Novartis, paid through Dartmouth College to support the costs of this study.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the participating institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Coker, S.A., Hurwitz, H.I., Sharma, S. et al. The effects of lapatinib on cardiac repolarization: results from a placebo controlled, single sequence, crossover study in patients with advanced solid tumors. Cancer Chemother Pharmacol 84, 383–392 (2019). https://doi.org/10.1007/s00280-019-03880-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03880-9