Abstract
Purpose
The therapeutic outcomes of stage III gastric cancer patient receiving D2 gastrectomy and adjuvant chemotherapy remain unsatisfactory. To improve the long-term outcomes in this population, the combination of docetaxel and S-1 (DS) therapy can be expected to be a useful regimen as neoadjuvant chemotherapy (NAC). This study aimed to prospectively evaluate the efficacy of NAC-DS for clinical stage III gastric cancer.
Methods
Between January 2010 and December 2013, 26 patients were enrolled. Patients with clinical stage III gastric cancer received two courses of docetaxel 40 mg/m2 on day 1, 15 and S-1 40 mg/m2 bid orally on day 1–7, 15–21 every 4 weeks, followed by radical D2 gastrectomy. Short- and long-term outcomes were evaluated. This study was approved by the ethics committee of Yokohama City University, and was registered in the University Hospital Medical Information Network (UMIN) database (ID: 000011521).
Results
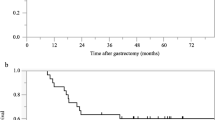
Of 26 patients, 24 (92.3%) patients completed two courses of NAC. After NAC-DS, Grade 3 neutropenia was observed in 5 (19.2%) patients including one patient with febrile neutropenia, anemia in 1 (3.8%) patient and diarrhea in 1 (3.8%) patient. All patients underwent R0 gastrectomy and pathological response was found in 15 (57.6%) patients. Postoperatively, Clavien-Dindo grade II complication occurred in 8 (30.7%) patients and no mortality was observed. The 5-year overall survival (OS) was 57.7%, median OS was 78.7 months and recurrence free survival (RFS) was 49.0%, median RFS was 45.4 months with 66.5 months median follow-up. Pathological response (HR = 0.091, 95% CI 0.011–0.730, p = 0.016) and > 5% body weight loss before NAC-DS (HR = 0.133, 95% CI 0.023–0.765, p = 0.024) were independent risk factors for recurrence, > 5% body weight loss before NAC-DS (HR = 0.133, 95% CI 0.023–0.765, p = 0.024) were independent risk factors for overall survival by multivariate analysis.
Conclusions
NAC-DS demonstrated acceptable toxicity with a high R0 resection rate in clinical stage III gastric cancer patients, especially in patients with good nutritional status. Further prospective study is warranted to compare the long-term outcomes between with and without NAC-DS.

Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–E386
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y (2011) Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 29(33):4387–4393
Park I, Ryu MH, Choi YH, Kang HJ, Yook JH, Park YS, Kim HJ, Jung HY, Lee GH, Kim KC, Kim BS, Kang YK (2013) A phase II study of neoadjuvant docetaxel, oxaliplatin, and S-1 (DOS) chemotherapy followed by surgery and adjuvant S-1 chemotherapy in potentially resectable gastric or gastroesophageal junction adenocarcinoma. Cancer Chemother Pharmacol 72(4):815–823
Hirakawa M, Sato Y, Ohnuma H, Takayama T, Sagawa T, Nobuoka T, Harada K, Miyamoto H, Sato Y, Takahashi Y, Katsuki S, Hirayama M, Takahashi M, Ono M, Maeda M, Takada K, Hayashi T, Sato T, Miyanishi K, Takimoto R, Kobune M, Hirata K, Kato J (2013) A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol 71(3):789–797
Oki E, Emi Y, Kusumoto T, Sakaguchi Y, Yamamoto M, Sadanaga N, Shimokawa M, Yamanaka T, Saeki H, Morita M, Takahashi I, Hirabayashi N, Sakai K, Orita H, Aishima S, Kakeji Y, Yamaguchi K, Yoshida K, Baba H, Maehara Y (2014) Phase II study of docetaxel and S-1 (DS) as neoadjuvant chemotherapy for clinical stage III resectable gastric cancer. Ann Surg Oncol 21(7):2340–2346
Yoshikawa T, Morita S, Tanabe K, Nishikawa K, Ito Y, Matsui T, Fujitani K, Kimura Y, Fujita J, Aoyama T, Hayashi T, Cho H, Tsuburaya A, Miyashita Y, Sakamoto J (2016) Survival results of a randomised two-by-two factorial phase II trial comparing neoadjuvant chemotherapy with two and four courses of S-1 plus cisplatin (SC) and paclitaxel plus cisplatin (PC) followed by D2 gastrectomy for resectable advanced gastric cancer. Eur J Cancer 62:103–111
Migita K, Nashimoto A, Yabusaki H, Matsuki A, Aizawa M (2016) Efficacy of neoadjuvant chemotherapy with docetaxel, cisplatin and S-1 for resectable locally advanced gastric cancer. Int J Clin Oncol 21(1):102–109
Sasaki K, Onodera S, Otsuka K, Satomura H, Kurayama E, Kubo T, Takahashi M, Ito J, Nakajima M, Yamaguchi S, Miyachi K, Kato H (2017) Validity of neoadjuvant chemotherapy with docetaxel, cisplatin, and S-1 for resectable locally advanced gastric cancer. Med Oncol 34(8):139
Kosaka T, Akiyama H, Makino H, Takagawa R, Kimura J, Ono H, Kunisaki C, Endo I (2014) Preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol 73(2):281–285
Japanese Gastric Cancer Association (2011) Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14(2):101–112
Shapiro J, van Lanschot JJB, Hulshof MCCM, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, Cuesta MA, Blaisse RJB, Busch ORC, Ten Kate FJW, Creemers GM, Punt CJA, Plukker JTM, Verheul HMW, Bilgen EJS, van Dekken H, van der Sangen MJC, Rozema T, Biermann K, Beukema JC, Piet AHM, van Rij CM, Reinders JG, Tilanus HW, Steyerberg EW, van der Gaast A, CROSS study group (2015) Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 16(9):1090–1098
Kurokawa Y, Hamakawa T, Miyazaki Y, Takahashi T, Yamasaki M, Miyata H, Nakajima K, Takiguchi S, Mori M, Doki Y (2015) Preoperative systemic and intraperitoneal chemotherapy consisting of S-1, cisplatin and docetaxel in patients with marginally resectable gastric cancer. Anticancer Res 35(4):2223–2228
Kobayashi D, Kodera Y (2017) Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer 20(Suppl 1):111–121
An JY, Kim HI, Cheong JH, Hyung WJ, Kim CB, Noh SH (2013) Pathologic and oncologic outcomes in locally advanced gastric cancer with neoadjuvant chemotherapy or chemoradiotherapy. Yonsei Med J 54(4):888–894
Kitayama J, Ishigami H, Yamaguchi H, Yamashita H, Emoto S, Kaisaki S, Watanabe T (2014) Salvage gastrectomy after intravenous and intraperitoneal paclitaxel (PTX) administration with oral S-1 for peritoneal dissemination of advanced gastric cancer with malignant ascites. Ann Surg Oncol 21(2):539–546
Yoshikawa T, Sasako M, Yamamoto S, Sano T, Imamura H, Fujitani K, Oshita H, Ito S, Kawashima Y, Fukushima (2009) Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. NBr J Surg 96(9):1015–1022
Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, Nakagawara H, Tajima H, Fujita H, Takamura H, Ninomiya I, Kitagawa H, Tani T, Fujimura T, Ohta T (2012) Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol 105(6):535–541
Iwasaki Y, Sasako M, Yamamoto S, Nakamura K, Sano T, Katai H, Tsujinaka T, Nashimoto A, Fukushima N, Tsuburaya A, Gastric Cancer Surgical Study Group of Japan Clinical Oncology Group (2013) Phase II study of preoperative chemotherapy with S-1 and cisplatin followed by gastrectomy for clinically resectable type 4 and large type 3 gastric cancers (JCOG0210). J Surg Oncol 107(7):741–745
Spolverato G, Ejaz A, Kim Y, Squires MH, Poultsides GA, Fields RC, Schmidt C, Weber SM, Votanopoulos K, Maithel SK, Pawlik TM (2014) Rates and patterns of recurrence after curative intent resection for gastric cancer: A United States multi-institutional analysis. J Am Coll Surg 219(4):664–675
Chang JS, Kim KH, Keum KC, Noh SH, Lim JS, Kim HS, Rha SY, Lee YC, Hyung WJ, Koom WS (2016) Recursive partition analysis of peritoneal and systemic recurrence in patients with gastric cancer who underwent D2 gastrectomy: Implications for neoadjuvant therapy consideration. J Surg Oncol 114(7):859–864
Kawamura Y, Satoh S, Umeki Y, Ishida Y, Suda K, Uyama I (2016) Evaluation of the recurrence pattern of gastric cancer after laparoscopic gastrectomy with D2 lymphadenectomy. Springerplus 5(1):821
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, Cho H, Tsuburaya A (2012) Risk factors for peritoneal recurrence in stage II/III gastric cancer patients who received S-1 adjuvant chemotherapy after D2 gastrectomy. Ann Surg Oncol 19(5):1568–1574
Jacquet P, Sugarbaker PH (1996) Peritoneal-plasma barrier. Cancer Treat Res 82:53–63
Tsuburaya A, Mizusawa J, Tanaka Y, Fukushima N, Nashimoto A, Sasako M, Stomach Cancer Study Group of the Japan Clinical Oncology Group (2014) Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg 101(6):653–660
Wang Y, Yu YY, Li W, Feng Y, Hou J, Ji Y, Sun YH, Shen KT, Shen ZB, Qin XY, Liu TS (2014) A phase II trial of Xeloda and oxaliplatin (XELOX) neo-adjuvant chemotherapy followed by surgery for advanced gastric cancer patients with para-aortic lymph node metastasis. Cancer Chemother Pharmacol 73(6):1155–1161
Wang X, Zhao L, Liu H, Zhong D, Liu W, Shan G, Dong F, Gao W, Bai C, Li X (2016) A phase II study of a modified FOLFOX6 regimen as neoadjuvant chemotherapy for locally advanced gastric cancer. Br J Cancer 114(12):1326–1333
Ito S, Sano T, Mizusawa J, Takahari D, Katayama H, Katai H, Kawashima Y, Kinoshita T, Terashima M, Nashimoto A, Nakamori M, Onaya H, Sasako M (2017) A phase II study of preoperative chemotherapy with docetaxel, cisplatin, and S-1 followed by gastrectomy with D2 plus para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis: JCOG1002. Gastric Cancer 20(2):322–331
Kurokawa Y, Shibata T, Sasako M, Sano T, Tsuburaya A, Iwasaki Y, Fukuda H (2014) Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer 17(3):514–521
Tahara T, Shibata T, Okubo M, Yoshida D, Kawamura T, Horiguchi N, Ishizuka T, Nagasaka M, Nakagawa Y, Ohmiya N (2017) Histological evaluations of primary lesions are independently associated with prognosis in patients with gastric cancer who receive neoadjuvant chemotherapy. Oncol Lett 13(6):4892–4896
Heger U, Bader F, Lordick F, Burian M, Langer R, Dobritz M, Blank S, Bruckner T, Becker K, Herrmann K, Siewert JR, Ott K (2014) Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer 17(3):478–488
Fujitani K, Mano M, Hirao M, Kodama Y, Tsujinaka T (2012) Posttherapy nodal status, not graded histologic response, predicts survival after neoadjuvant chemotherapy for advanced gastric cancer. Ann Surg Oncol 19(6):1936–1943
Schmidt T, Sicic L, Blank S, Becker K, Weichert W, Bruckner T, Parakonthun T, Langer R, Büchler MW, Siewert JR, Lordick F, Ott K (2014) Prognostic value of histopathological regression in 850 neoadjuvantly treated oesophagogastric adenocarcinomas. Br J Cancer 110(7):1712–1720
Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H (2003) Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer 98(7):1521–1530
Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G (1994) Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathol Correlat Cancer 73(11):2680–2686
Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, Jatoi A, Kalantar-Zadeh K, Lochs H, Mantovani G, Marks D, Mitch WE, Muscaritoli M, Najand A, Ponikowski P, Rossi Fanelli F, Schambelan M, Schols A, Schuster M, Thomas D, Wolfe R, Anker SD (2008) Cachexia: a new definition. Clin Nutr 27(6):793–799
Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, Radbruch L, Ravasco P, Walsh D, Wilcock A, Kaasa S, Baracos VE (2011) Definition and classification of cancer cachexia: an international consensus. Lancet Oncol 12(5):489–495
Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, Cohen MH, Douglass HO Jr, Engstrom PF, Ezdinli EZ, Horton J, Johnson GJ, Moertel CG, Oken MM, Perlia C, Rosenbaum C, Silverstein MN, Skeel RT, Sponzo RW, Tormey DC (1980) Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 69(4):491–497
Langius JA, Bakker S, Rietveld DH, Kruizenga HM, Langendijk JA, Weijs PJ, Leemans CR (2013) Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br J Cancer 109(5):1093–1099
van der Schaaf MK, Tilanus HW, van Lanschot JJ, Johar AM, Lagergren P, Lagergren J, Wijnhoven BP (2014) The influence of preoperative weight loss on the postoperative course after esophageal cancer resection. J Thorac Cardiovasc Surg 147(1):490–495
Liu X, Qiu H, Kong P, Zhou Z, Sun X (2017) Gastric cancer, nutritional status, and outcome. Onco Targets Ther 10:2107–2114
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kosaka, T., Akiyama, H., Miyamoto, H. et al. Outcomes of preoperative S-1 and docetaxel combination chemotherapy in patients with locally advanced gastric cancer. Cancer Chemother Pharmacol 83, 1047–1055 (2019). https://doi.org/10.1007/s00280-019-03813-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03813-6