Abstract
Purpose
The role of carbohydrate antigen 19-9 (CA19-9) kinetics in patients with biliary tract cancer (BTC) receiving chemotherapy remains to be elucidated.
Methods
A total of 185 advanced or recurrent BTC patients receiving a first line chemotherapy between January 2006 and March 2016, were retrospectively studied. Serum CA19-9 was measured at baseline and after two cycles of chemotherapy, and patients were categorized based on CA19-9 response: CA19-9 decrease group (≥ 30% decrease), stable group (< 30% decrease and < 20% increase) and increase group (≥ 20% increase). The associations of CA19-9 response with radiological tumor response, progression-free survival (PFS) and overall survival (OS) were investigated.
Results
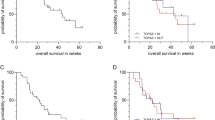
There was a statistically significant association between CA19-9 response and radiological tumor responses (p < 0.001). The median PFS and OS were significantly different among three groups according to CA19-9 response: PFS of 8.0, 5.7 and 3.5 months in CA19-9 decrease, stable and increase groups (p < 0.001) and OS of 18.8, 16.0 and 7.5 months in CA19-9 decrease, stable and increase groups, respectively (p < 0.001). Multivariate analyses showed that CA19-9 response was prognostic both of OS and PFS in addition, to CA19-9 at baseline, and performance status.
Conclusion
CA19-9 kinetics after the first two cycles of a first line chemotherapy was a prognostic factor for OS and PFS in patients with advanced and recurrent BTC.



Similar content being viewed by others
Abbreviations
- BTC:
-
Biliary tract cancer
- CA19-9:
-
Carbohydrate antigen 19-9
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
References
Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics (2017). CA Cancer J Clin 67:7–30
Glimelius B, Hoffman K, Sjoden PO et al (1996) Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol 7:593–600
Okusaka T, Nakachi K, Fukutomi A et al (2010) Gemcitabine alone or in combination with cisplatin in patients with biliary tract cancer: a comparative multicentre study in Japan. Br J Cancer 103:469–474
Valle J, Wasan H, Palmer DH et al (2010) Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med 362:1273–1281
Takahara N, Isayama H, Nakai Y et al (2017) Gemcitabine and S-1 versus gemcitabine and cisplatin treatment in patients with advanced biliary tract cancer: a multicenter retrospective study. Invest New Drugs 35:269–276
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Pectasides D, Mylonakis A, Kostopoulou M et al (1997) CEA, CA19-9, and CA-50 in monitoring gastric carcinoma. Am J Clin Oncol 20:348–353
Forones NM, Tanaka M (1999) CEA and CA19-9 as prognostic indexes in colorectal cancer. Hepatogastroenterology 46:905–908
Sato Y, Fujimoto D, Uehara K et al (2016) The prognostic value of serum CA19-9 for patients with advanced lung adenocarcinoma. BMC Cancer 16:890
Santotoribio JD, Garcia-de la Torre A, Canavate-Solano C et al (2016) Cancer antigens 19.9 and 125 as tumor markers in patients with mucinous ovarian tumors. Eur J Gynaecol Oncol 37:26–29
Elisei R, Lorusso L, Piaggi P et al (2015) Elevated level of serum carbohydrate antigen 19.9 as predictor of mortality in patients with advanced medullary thyroid cancer. Eur J Endocrinol 173:297–304
Nakai Y, Kawabe T, Isayama H et al (2008) CA19-9 response as an early indicator of the effectiveness of gemcitabine in patients with advanced pancreatic cancer. Int Soc Cell 75:120–126
Nakai Y, Isayama H, Sasaki T et al (2013) A retrospective analysis of early CA199 change in salvage chemotherapy for refractory pancreatic cancer. Cancer Chemother Pharmacol 72:1291–1297
Chiorean EG, Von Hoff DD, Reni M et al (2016) CA19-9 decrease at 8 weeks as a predictor of overall survival in a randomized phase III trial (MPACT) of weekly nab-paclitaxel plus gemcitabine versus gemcitabine alone in patients with metastatic pancreatic cancer. Ann Oncol 27:654–660
Chen Y, Shao Z, Chen W et al (2017) A varying-coefficient cox model for the effect of CA19-9 kinetics on overall survival in patients with advanced pancreatic cancer. Oncotarget 8:29925–29934
Tempero MA, Uchida E, Takasaki H et al (1987) Relationship of carbohydrate antigen 19-9 and Lewis antigens in pancreatic cancer. Cancer Res 47:5501–5503
Sasaki T, Isayama H, Nakai Y et al (2010) Multicenter, phase II study of gemcitabine and S-1 combination chemotherapy in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol 65:1101–1107
Sasaki T, Isayama H, Nakai Y et al (2013) A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol 71:973–979
Sasaki T, Isayama H, Yashima Y et al (2009) S-1 monotherapy in patients with advanced biliary tract cancer. Int Soc Cell 77:71–74
Takahara N, Isayama H, Nakai Y, Ioka T, Kanai M, Sasaki T, Furuse J, Koike K (2017) A single arm, prospective multicenter phase II study FOLFIRINOX in patients with advanced and recurrent biliary tract cancer. J Clin Oncol. 35, no. 4_suppl - published online before print
Buyse M, Piedbois P (1996) On the relationship between response to treatment and survival time. Stat Med 15:2797–2812
Harder J, Kummer O, Olschewski M et al (2007) Prognostic relevance of carbohydrate antigen 19-9 levels in patients with advanced biliary tract cancer. Cancer Epidemiol Biomarkers Prev 16:2097–2100
Fornaro L, Cereda S, Aprile G et al (2014) Multivariate prognostic factors analysis for second-line chemotherapy in advanced biliary tract cancer. Br J Cancer 110:2165–2169
Grunnet M, Christensen IJ, Lassen U et al (2015) Decline in CA19-9 during chemotherapy predicts survival in four independent cohorts of patients with inoperable bile duct cancer. Eur J Cancer 51:1381–1388
Lee DW, Im SA, Kim YJ et al. CA19-9 or CEA decline after the first Cycle of treatment predicts survival in advanced biliary tract cancer patients treated with S-1 and cisplatin chemotherapy. Cancer Res Treat 2017
Lee BS, Lee SH, Son JH et al (2016) Prognostic value of CA19-9 kinetics during gemcitabine-based chemotherapy in patients with advanced cholangiocarcinoma. J Gastroenterol Hepatol 31:493–500
Cho KM, Park H, Oh DY et al (2017) Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and their dynamic changes during chemotherapy is useful to predict a more accurate prognosis of advanced biliary tract cancer. Oncotarget 8:2329–2341
Acknowledgements
N.T., Y.N., and H.I.: conception and design of the study; collection, analysis, and interpretation of data; drafting of the article. T.S.: conception and design of the study; collection, analysis, and interpretation of data; critical revision of the article for important intellectual content. K.S., K.N., T.S., T.N., T.S., K.I., R.H., T.T., and R.U.: conception and design of the study; collection, analysis, and interpretation of data. S.M., H.K., M. T.: critical revision of the article for important intellectual content. K.K.: critical revision of the article for important intellectual content, and supervision of the study. All authors: final approval of the article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was not funded by any foundation, company, industry, or external source.
Conflict of interest
All authors declare no conflict of interest related to this article.
Ethical approval
This study was in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Takahara, N., Nakai, Y., Isayama, H. et al. CA19-9 kinetics during systemic chemotherapy in patients with advanced or recurrent biliary tract cancer. Cancer Chemother Pharmacol 80, 1105–1112 (2017). https://doi.org/10.1007/s00280-017-3456-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3456-9