Abstract
Purpose
Transforming growth factor-beta inhibitors may enhance the antitumor activity of gemcitabine with acceptable safety and tolerability. This open-label, multicenter, non-randomized phase 1b study assessed the safety/tolerability, pharmacokinetics, and tumor response of galunisertib plus gemcitabine in Japanese patients with advanced or metastatic pancreatic cancer.
Methods
During each 28-day cycle, galunisertib 150 mg was administered orally twice daily (300 mg/day) for 14 days, followed by 14 days of rest. Gemcitabine 1000 mg/m2 was intravenously given on Days 8, 15, and 22. Safety was assessed by the incidence of dose-limiting toxicities (DLTs) in the first cycle and treatment-emergent adverse events (TEAEs). Efficacy was assessed by antitumor activity and changes in carbohydrate antigen 19-9 (CA19-9).
Results
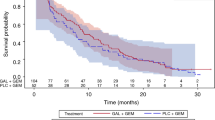
No DLTs were reported. All 7 enrolled patients had ≥1 TEAE, of which the most common included anorexia, decreased neutrophil count, and decreased white blood cell count. Grade ≥3 TEAEs were observed in 6 patients; 4 patients had Grade ≥3 TEAEs (decreased neutrophil, white blood cell, and lymphocyte count; hypophosphatemia) considered possibly related to study drug(s). The pharmacokinetic profile of galunisertib in combination with gemcitabine was similar to that previously observed for galunisertib alone. The clinical response [complete response (CR), partial response (PR), or stable disease] rate was 42.9%, and the median progression-free survival was 64 days; no CR/PR were achieved.
Conclusion
Galunisertib plus gemcitabine had an acceptable safety/tolerability profile with evidence of efficacy in Japanese patients with advanced or metastatic pancreatic cancer.


Similar content being viewed by others
References
Cancer Information Service NCC, Japan (2016) Cancer registry and statistics. http://ganjoho.jp/data/reg_stat/statistics/dl/cancer_mortality(1958-2014).xls. Accessed 30 September 2016
Center for Cancer Control and Information Services NCC (2016) Monitoring of cancer incidence in Japan—Survival 2006–2008 Report. http://ganjoho.jp/data/reg_stat/statistics/dl/cancer_survival(1993-2008).xls. Accessed 30 September 2016
Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, Mohile SG, Mumber M, Schulick R, Shapiro M, Urba S, Zeh HJ, Katz MHG (2016) Potentially curable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34:2541–2556. doi:10.1200/jco.2016.67.5553
Sohal DPS, Mangu PB, Khorana AA, Shah MA, Philip PA, O’Reilly EM, Uronis HE, Ramanathan RK, Crane CH, Engebretson A, Ruggiero JT, Copur MS, Lau M, Urba S, Laheru D (2016) Metastatic pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34:2784–2796. doi:10.1200/jco.2016.67.1412
Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, Javle MM, Eads JR, Allen P, Ko AH, Engebretson A, Herman JM, Strickler JH, Benson AB, Urba S, Yee NS (2016) Locally advanced, unresectable pancreatic cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34:2654–2668. doi:10.1200/jco.2016.67.5561
Herbertz S, Sawyer JS, Stauber AJ, Gueorguieva I, Driscoll KE, Estrem ST, Cleverly AL, Desaiah D, Guba SC, Benhadji KA, Slapak CA, Lahn MM (2015) Clinical development of galunisertib (LY2157299 monohydrate), a small molecule inhibitor of transforming growth factor-beta signaling pathway. Drug Des Devel Ther 9:4479–4499. doi:10.2147/DDDT.S86621
Rodon J, Carducci MA, Sepulveda-Sánchez JM, Azaro A, Calvo E, Seoane J, Braña I, Sicart E, Gueorguieva I, Cleverly AL, Pillay NS, Desaiah D, Estrem ST, Paz-Ares L, Holdhoff M, Blakeley J, Lahn MM, Baselga J (2015) First-in-human dose study of the novel transforming growth factor-β receptor I kinase inhibitor LY2157299 monohydrate in patients with advanced cancer and glioma. Clin Cancer Res 21:553–560. doi:10.1158/1078-0432.ccr-14-1380
Faivre SJSA, Kelley RK, Merle P, Gane E, Douillard JY, Waldschmidt D, Mulcahy MF, Costentin C, Minguez B, Papappicco P, Gueorguieva I, Cleverly A, Desaiah D, Lahn MM, Ameryckx S, Benhadji KA, Raymond E, Giannelli G (2014) A phase 2 study of a novel transforming growth factor-beta (TGF-β1) receptor I kinase inhibitor, LY2157299 monohydrate (LY), in patients with advanced hepatocellular carcinoma (HCC). J Clin Oncol 32(suppl 3):Abstract LBA173
Fujiwara Y, Nokihara H, Yamada Y, Yamamoto N, Sunami K, Utsumi H, Asou H, TakahashI O, Ogasawara K, Gueorguieva I, Tamura T (2015) Phase 1 study of galunisertib, a TGF-beta receptor I kinase inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 76:1143–1152. doi:10.1007/s00280-015-2895-4
Miyazono K (2000) TGF-β/SMAD signaling and its involvement in tumor progression. Biol Pharm Bull 23:1125–1130. doi:10.1248/bpb.23.1125
Melisi D, Ishiyama S, Sclabas GM, Fleming JB, Xia Q, Tortora G, Abbruzzese JL, Chiao PJ (2008) LY2109761, a novel transforming growth factor β receptor type I and type II dual inhibitor, as a therapeutic approach to suppressing pancreatic cancer metastasis. Mol Cancer Ther 7:829–840. doi:10.1158/1535-7163.mct-07-0337
Schlingensiepen K-H, Stauder G, Bischof A, Egger T, Hafner M, Herrmuth H, Kielmanowicz M, Jachimczak P (2004) TGF-beta2 suppression in pancreatic cancer by the antisense oligonucleotide AP 12009: preclinical efficacy data. Proc Am Assoc Cancer Res 45(suppl):Abstract 2955
Kozloff MCR, Nadal T, Gueorguieva I, Cleverly A, Desaiah D, Lahn MMF, Pillay S, Blunt A, Tabernero J, Macarulla T (2013) Phase Ib study evaluating safety and pharmacokinetics (PK) of the oral transforming growth factor-beta (TGF-β) receptor I kinase inhibitor LY2157299 monohydrate (LY) when combined with gemcitabine in patients with advanced cancer. J Clin Oncol 31(suppl):Abstract 2563
Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Gueorguieva I, Desaiah D, Lahn MM, Blunt A, Benhadji KA, Tabernero J (2016) A randomized phase II, double-blind study to evaluate the efficacy and safety of galunisertib + gemcitabine (GG) or gemcitabine + placebo (GP) in patients with unresectable pancreatic cancer (PC). Cancer Res 76(suppl 14):Abstract CT068. doi:10.1158/1538-7445.am2016-ct068
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247. doi:10.1016/j.ejca.2008.10.026
International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (2005) The clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for nonantiarrhythmic drugs. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E14/E14_Guideline.pdf. Accessed 20 Jan 2017
Acknowledgements
The authors would like to thank all study participants and their families. We also wish to thank the study team members at the study centers, Eli Lilly and Company, and the CROs.
Author information
Authors and Affiliations
Contributions
All authors participated in the interpretation of study results, and in the drafting, critical revision, and approval of the final version of the manuscript. MI, ML, KO, and HU were involved in the study design and data analyses. MI and HU were involved in data collection. MI, HT, SK, and HU were investigators in the study, and KO conducted the pharmacokinetic analysis. KB was involved in the analysis and interpretation of data.
Corresponding author
Ethics declarations
Funding
This study was sponsored by Eli Lilly Japan, manufacturer/licensee of galunisertib (LY2157299 monohydrate, Grant Number H9H-JE-JBAO). Medical writing assistance was provided by Mark Snape, MB BS, CMPP and Hiroko Ebina, BPharm, Ph, MBA of ProScribe—Envision Pharma Group, and was funded by Eli Lilly and Company. ProScribe’s services complied with international guidelines for Good Publication Practice (GPP3). Eli Lilly Japan was involved in the study design, data collection, data analysis, and preparation of the manuscript.
Conflict of interest
KB/HF and ML/KO are current and former employees of Eli Lilly and Company, respectively. ML, KB, and HF own shares in Eli Lilly and Company. MI has received research funding from Taiho and Eli Lilly and Company, SK has received research funding from Eli Lilly and Company, Pfizer, Aslan Pharmaceuticals, AstraZeneca and Bayer, and HU has received research funding from Taiho and Eli Lilly and Company, and has been involved in a speakers’ bureau with Taiho. HT has no conflicts of interest to declare.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Ikeda, M., Takahashi, H., Kondo, S. et al. Phase 1b study of galunisertib in combination with gemcitabine in Japanese patients with metastatic or locally advanced pancreatic cancer. Cancer Chemother Pharmacol 79, 1169–1177 (2017). https://doi.org/10.1007/s00280-017-3313-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-017-3313-x