Abstract
Purpose
Cks1, a conformationally heterogenous 9 kDa protein, is markedly overexpressed in cancer cells and contributes to tumor development. Cks1 is an essential component of the SCF-Skp2 ubiquitin ligase complex that targets the Cdk inhibitors p27Kip1 and p21Cip1. Cks1 is known to interact with the Hsp90-Cdc37 chaperone machinery, although whether this facilitates its conformational maturation and stability is not known. To test whether abrogating the chaperone function of Hsp90 could destabilize Cks1, we examined the effects of treating different cancer cell lines with the benzoquinone ansamycin 17-allylamino geldanamycin (17-AAG), a compound that selectively binds Hsp90 and potently inhibits its ATP-dependent chaperone activity.
Methods
The effect of Hsp90 inhibition using 17-AAG on Cks1 protein and associated cell cycle proteins including Skp2, p27Kip1, p21Cip1, and Cdk1 in cancer cells was determined by Western blotting. Ubiquitination analysis was carried out by transfecting cells with an HA-ubiquitin plasmid and specifically immunoprecipitating Cks1 to examine polyubiquitinated species. Flow cytometry was utilized to examine the effects of Hsp90 inhibition on cell cycle profiles.
Results
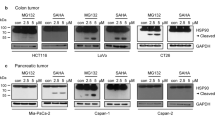
Here, we demonstrate for the first time that inhibition of Hsp90 utilizing 17-AAG destabilizes Cks1 in cancer cells by promoting its ubiquitination and proteasomal degradation. 17-AAG-induced Cks1 depletion was accompanied by concomitant decreases in Skp2 and Cdk1. 17-AAG treatment also induced G2/M accumulation in MCF-7 breast carcinoma cells, and G1 accumulation in the colon carcinoma lines HCT116 and SW620.
Conclusions
We conclude that perturbing the Hsp90 pathway could provide a useful therapeutic strategy in tumors driven by Cks1 overexpression.






Similar content being viewed by others
References
Khattar V, Thottassery JV (2013) Cks1: structure, emerging roles and implications in multiple cancers. J Cancer Ther 4(8):1341–1354
Westbrook L, Ramanathan HN, Isayeva T, Mittal AR, Qu Z, Johnson MD, Kern FG, Ponnazhagan S, Grubbs CJ, Thottassery JV (2009) High Cks1 expression in transgenic and carcinogen-initiated mammary tumors is not always accompanied by reduction in p27Kip1. Int J Oncol 34(5):1425–1431
Slotky M, Shapira M, Ben-Izhak O, Linn S, Futerman B, Tsalic M, Hershko DD (2005) The expression of the ubiquitin ligase subunit Cks1 in human breast cancer. Breast Cancer Res BCR 7(5):R737–R744
Shapira M, Ben-Izhak O, Slotky M, Goldin O, Lahav-Baratz S, Hershko DD (2006) Expression of the ubiquitin ligase subunit cyclin kinase subunit 1 and its relationship to S-phase kinase protein 2 and p27Kip1 in prostate cancer. J Urol 176(5):2285–2289
Hershko DD, Shapira M (2006) Prognostic role of p27Kip1 deregulation in colorectal cancer. Cancer 107(4):668–675
Urbanowicz-Kachnowicz I, Baghdassarian N, Nakache C, Gracia D, Mekki Y, Bryon PA, Ffrench M (1999) Ckshs expression is linked to cell proliferation in normal and malignant human lymphoid cells. Int J Cancer 82(1):98–104
Tsai YS, Chang HC, Chuang LY, Hung WC (2005) RNA silencing of Cks1 induced G2/M arrest and apoptosis in human lung cancer cells. IUBMB Life 57(8):583–589
Chang H, Jiang N, Jiang H, Saha MN, Qi C, Xu W, Reece D (2010) CKS1B nuclear expression is inversely correlated with p27Kip1 expression and is predictive of an adverse survival in patients with multiple myeloma. Haematologica 95(9):1542–1547
Westbrook L, Manuvakhova M, Kern FG, Estes NR 2nd, Ramanathan HN, Thottassery JV (2007) Cks1 regulates cdk1 expression: a novel role during mitotic entry in breast cancer cells. Cancer Res 67(23):11393–11401
Ganoth D, Bornstein G, Ko TK, Larsen B, Tyers M, Pagano M, Hershko A (2001) The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat Cell Biol 3(3):321–324
Spruck C, Strohmaier H, Watson M, Smith AP, Ryan A, Krek TW, Reed SI (2001) A CDK-independent function of mammalian Cks1: targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol Cell 7(3):639–650
Liberal V, Martinsson-Ahlzen HS, Liberal J, Spruck CH, Widschwendter M, McGowan CH, Reed SI (2012) Cyclin-dependent kinase subunit (Cks) 1 or Cks2 overexpression overrides the DNA damage response barrier triggered by activated oncoproteins. Proc Natl Acad Sci USA 109(8):2754–2759
Keller UB, Old JB, Dorsey FC, Nilsson JA, Nilsson L, MacLean KH, Chung L, Yang C, Spruck C, Boyd K, Reed SI, Cleveland JL (2007) Myc targets Cks1 to provoke the suppression of p27Kip1, proliferation and lymphomagenesis. EMBO J 26(10):2562–2574
Lee EK, Kim DG, Kim JS, Yoon Y (2011) Cell-cycle regulator Cks1 promotes hepatocellular carcinoma by supporting NF-kappaB-dependent expression of interleukin-8. Cancer Res 71(21):6827–6835
Wang XC, Tian LL, Tian J, Wu HL, Meng AM (2009) Overexpression of Cks1 is associated with poor survival by inhibiting apoptosis in breast cancer. J Cancer Res Clin Oncol 135(10):1393–1401
Khattar V, Thottassery JV (2015) Cks1 proteasomal turnover is a predominant mode of regulation in breast cancer cells: role of key tyrosines and lysines. Int J Oncol 46(1):395–406
Hattori T, Kitagawa K, Uchida C, Oda T, Kitagawa M (2003) Cks1 is degraded via the ubiquitin-proteasome pathway in a cell cycle-dependent manner. Genes Cells 8(11):889–896
Bashir T, Dorrello NV, Amador V, Guardavaccaro D, Pagano M (2004) Control of the SCF(Skp2-Cks1) ubiquitin ligase by the APC/C(Cdh1) ubiquitin ligase. Nature 428(6979):190–193
Trepel J, Mollapour M, Giaccone G, Neckers L (2010) Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer 10(8):537–549
Whitesell L, Lindquist SL (2005) HSP90 and the chaperoning of cancer. Nat Rev Cancer 5(10):761–772
Kamal A, Thao L, Sensintaffar J, Zhang L, Boehm MF, Fritz LC, Burrows FJ (2003) A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425(6956):407–410
Hohfeld J, Cyr DM, Patterson C (2001) From the cradle to the grave: molecular chaperones that may choose between folding and degradation. EMBO Rep 2(10):885–890
McClellan AJ, Tam S, Kaganovich D, Frydman J (2005) Protein quality control: chaperones culling corrupt conformations. Nat Cell Biol 7(8):736–741
Krishnamoorthy GP, Guida T, Alfano L, Avilla E, Santoro M, Carlomagno F, Melillo RM (2013) Molecular mechanism of 17-allylamino-17-demethoxygeldanamycin (17-AAG)-induced AXL receptor tyrosine kinase degradation. J Biol Chem 288(24):17481–17494
Rousseau F, Schymkowitz JW, Wilkinson HR, Itzhaki LS (2004) Intermediates control domain swapping during folding of p13suc1. J Biol Chem 279(9):8368–8377
Seeliger MA, Spichty M, Kelly SE, Bycroft M, Freund SM, Karplus M, Itzhaki LS (2005) Role of conformational heterogeneity in domain swapping and adapter function of the Cks proteins. J Biol Chem 280(34):30448–30459
Schneider C, Sepp-Lorenzino L, Nimmesgern E, Ouerfelli O, Danishefsky S, Rosen N, Hartl FU (1996) Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc Natl Acad Sci USA 93(25):14536–14541
Radulovic M, Crane E, Crawford M, Godovac-Zimmermann J, Yu VP (2010) CKS proteins protect mitochondrial genome integrity by interacting with mitochondrial single-stranded DNA-binding protein. Mol Cell Prote 9(1):145–152
Wang W, Ungermannova D, Jin J, Harper JW, Liu X (2004) Negative regulation of SCFSkp2 ubiquitin ligase by TGF-beta signaling. Oncogene 23(5):1064–1075
Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A (2003) Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278(28):25752–25757
Bagatell R, Khan O, Paine-Murrieta G, Taylor CW, Akinaga S, Whitesell L (2001) Destabilization of steroid receptors by heat shock protein 90-binding drugs: a ligand-independent approach to hormonal therapy of breast cancer. Clin Cancer Res 7(7):2076–2084
Watanabe G, Behrns KE, Kim JS, Kim RD (2009) Heat shock protein 90 inhibition abrogates hepatocellular cancer growth through cdc2-mediated G2/M cell cycle arrest and apoptosis. Cancer Chemother Pharmacol 64(3):433–443
Wang JJ, Fang ZX, Ye HM, You P, Cai MJ, Duan HB, Wang F, Zhang ZY (2013) Clinical significance of overexpressed cyclin-dependent kinase subunits 1 and 2 in esophageal carcinoma. Dis Esophagus 26(7):729–736
Lee SW, Kang SB, Lee DS, Lee JU (2013) Akt and Cks1 are related with lymph node metastasis in gastric adenocarcinoma. Hepatogastroenterology 60(124):932–937
Masuda TA, Inoue H, Nishida K, Sonoda H, Yoshikawa Y, Kakeji Y, Utsunomiya T, Mori M (2003) Cyclin-dependent kinase 1 gene expression is associated with poor prognosis in gastric carcinoma. Clin Cancer Res 9(15):5693–5698
Shapira M, Ben-Izhak O, Linn S, Futerman B, Minkov I, Hershko DD (2005) The prognostic impact of the ubiquitin ligase subunits Skp2 and Cks1 in colorectal carcinoma. Cancer 103(7):1336–1346
Shapira M, Ben-Izhak O, Bishara B, Futerman B, Minkov I, Krausz MM, Pagano M, Hershko DD (2004) Alterations in the expression of the cell cycle regulatory protein cyclin kinase subunit 1 in colorectal carcinoma. Cancer 100(8):1615–1621
Calvisi DF, Ladu S, Pinna F, Frau M, Tomasi ML, Sini M, Simile MM, Bonelli P, Muroni MR, Seddaiu MA, Lim DS, Feo F, Pascale RM (2009) SKP2 and CKS1 promote degradation of cell cycle regulators and are associated with hepatocellular carcinoma prognosis. Gastroenterology 137(5):1816–1826 e1811-1810
Moser C, Lang SA, Kainz S, Gaumann A, Fichtner-Feigl S, Koehl GE, Schlitt HJ, Geissler EK, Stoeltzing O (2007) Blocking heat shock protein-90 inhibits the invasive properties and hepatic growth of human colon cancer cells and improves the efficacy of oxaliplatin in p53-deficient colon cancer tumors in vivo. Mol Cancer Ther 6(11):2868–2878
Milicevic Z, Bogojevic D, Mihailovic M, Petrovic M, Krivokapic Z (2008) Molecular characterization of hsp90 isoforms in colorectal cancer cells and its association with tumour progression. Int J Oncol 32(6):1169–1178
Mimnaugh EG, Xu W, Vos M, Yuan X, Isaacs JS, Bisht KS, Gius D, Neckers L (2004) Simultaneous inhibition of hsp 90 and the proteasome promotes protein ubiquitination, causes endoplasmic reticulum-derived cytosolic vacuolization, and enhances antitumor activity. Mol Cancer Ther 3(5):551–566
Kaganovich D, Kopito R, Frydman J (2008) Misfolded proteins partition between two distinct quality control compartments. Nature 454(7208):1088–1095
Acknowledgments
JS is a fellow in the Howard Hughes Medical Institute Graduate Fellowship Program at UAB. Work in the authors’ laboratories was supported by grants from the Komen Breast Cancer Foundation grant (BCTR00-456, JVT), NCI Breast SPORE Developmental grant (JVT), and NIH grants ES016354 (BX) and CA133093 (BX).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khattar, V., Fried, J., Xu, B. et al. Cks1 proteasomal degradation is induced by inhibiting Hsp90-mediated chaperoning in cancer cells. Cancer Chemother Pharmacol 75, 411–420 (2015). https://doi.org/10.1007/s00280-014-2666-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2666-7