Abstract
Introduction
Taxane-based chemotherapy is one of the most commonly used agents in patients with advanced urothelial carcinoma who developed disease progression after gemcitabine and cisplatin combination chemotherapy. The response rates, however, are dismal reported around 10–20 %. Recently, promising efficacy results of paclitaxel when combined with oral cyclophosphamide as metronomic therapy have been reported.
Patients and methods
Patients received paclitaxel 175 mg/m2 intravenously over 3 h on day 1 and cyclophosphamide 50 mg/day orally on days 1–7 every 3 weeks. For patients with ECOG, performance status was two or previous radiotherapy on 25 % or more of their bone marrow, paclitaxel was given at a dose of 135 mg/m2 for the first cycle, followed by intra-patient dose escalation to 175 mg/m2 if clinically significant toxicities were not observed during the first cycle. Oral cyclophosphamide was administered for extended 3 weeks after the safety of 1-week metronomic therapy was confirmed. The primary end points were response rate (RECIST v.1.1) and progression-free survival.
Results
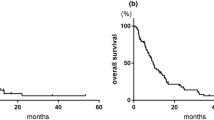
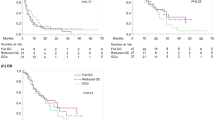
From March 2012 to March 2014, 46 patients with bladder or upper urinary tract cancer were treated with this regimen in our institution after failure to gemcitabine and cisplatin combination chemotherapy. After excluding four patients with pathologies other than urothelial carcinoma (one collecting duct carcinoma, two small cell carcinoma, and one squamous cell carcinoma), a total of 42 patients were included in this study. The platinum-free interval was <6 months in 33 (78.6 %) patients, and 39 (92.8 %) were categorized into the intermediate or poor prognosis group according to Bajorin’s risk model. The objective response rate was 33.3 % (n = 14) with a median response duration of 4.3 months. The median time to progression was 3.0 months (95 % CI 1.7–4.3 months), and the median OS was 6.3 months (95 % CI 4.6–8.0 months). The most frequent and clinically significant non-hematologic toxicities were peripheral sensory neuropathy (56 %), fatigue (35 %), and myalgia (28 %) in order, but none of them showed severity of grade 3 or more. Grade ≥ 3 neutropenia occurred only in two patients (6 %), and one of them developed febrile neutropenia. The duration of metronomic cyclophosphamide did not significantly affect the toxicity profile, and it could be safely administered for whole cycles.
Conclusion
Metronomic oral cyclophosphamide combined with paclitaxel appears to be both efficacious and safe as a salvage chemotherapy, particularly in heavily pretreated patients with advanced urothelial carcinoma after gemcitabine–cisplatin failure.

Similar content being viewed by others
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61(2):69–90. doi:10.3322/caac.20107
von der Maase H, Hansen SW, Roberts JT, Dogliotti L, Oliver T, Moore MJ, Bodrogi I, Albers P, Knuth A, Lippert CM, Kerbrat P, Sanchez Rovira P, Wersall P, Cleall SP, Roychowdhury DF, Tomlin I, Visseren-Grul CM, Conte PF (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18(17):3068–3077
Sternberg CN, de Mulder P, Schornagel JH, Theodore C, Fossa SD, van Oosterom AT, Witjes JA, Spina M, van Groeningen CJ, Duclos B, Roberts JT, de Balincourt C, Collette L (2006) Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42(1):50–54. doi:10.1016/j.ejca.2005.08.032
Roth BJ, Dreicer R, Einhorn LH, Neuberg D, Johnson DH, Smith JL, Hudes GR, Schultz SM, Loehrer PJ (1994) Significant activity of paclitaxel in advanced transitional-cell carcinoma of the urothelium: a phase II trial of the Eastern Cooperative Oncology Group. J Clin Oncol 12(11):2264–2270
Bajorin DF (2000) Paclitaxel in the treatment of advanced urothelial cancer. Oncology (Williston Park) 14(1):43–52, 57; discussion 58, 61–42
Raghavn D (2003) Progress in the chemotherapy of metastatic cancer of the urinary tract. Cancer 97(8 Suppl):2050–2055. doi:10.1002/cncr.11280
Dreicer R, Manola J, Roth BJ, Cohen MB, Hatfield AK, Wilding G (2000) Phase II study of cisplatin and paclitaxel in advanced carcinoma of the urothelium: an Eastern Cooperative Oncology Group study. J Clin Oncol 18(5):1058–1061
Sengeløv L, Kamby C, Lund B, Engelholm SA (1998) Docetaxel and cisplatin in metastatic urothelial cancer: a phase II study. J Clin Oncol 16(10):3392–3397
Joly F, Houédé N, Noal S, Chevreau C, Priou F, Chinet-Charrot P, Rolland F, Fléchon A, Henry-Amar M, Culine S (2009) Do patients with advanced urothelial carcinoma benefit from weekly paclitaxel chemotherapy? A GETUG phase II study. Clin Genitourin Cancer 7(2):E28–E33
Papamichael D, Gallagher CJ, Oliver RT, Johnson PW, Waxman J (1997) Phase II study of paclitaxel in pretreated patients with locally advanced/metastatic cancer of the bladder and ureter. Br J Cancer 75(4):606–607
Vaughn DJ, Broome CM, Hussain M, Gutheil JC, Markowitz AB (2002) Phase II trial of weekly paclitaxel in patients with previously treated advanced urothelial cancer. J Clin Oncol 20(4):937–940
McCaffrey JA, Hilton S, Mazumdar M, Sadan S, Kelly WK, Scher HI, Bajorin DF (1997) Phase II trial of docetaxel in patients with advanced or metastatic transitional-cell carcinoma. J Clin Oncol 15(5):1853–1857
Lee JL, Ahn JH, Park SH, Lim HY, Kwon JH, Ahn S, Song C, Hong JH, Kim CS, Ahn H (2012) Phase II study of a cremophor-free, polymeric micelle formulation of paclitaxel for patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Invest New Drugs 30(5):1984–1990. doi:10.1007/s10637-011-9757-7
Ko Y-J, Canil CM, Mukherjee SD, Winquist E, Elser C, Eisen A, Reaume MN, Zhang L, Sridhar SS (2013) Nanoparticle albumin-bound paclitaxel for second-line treatment of metastatic urothelial carcinoma: a single group, multicentre, phase 2 study. Lancet Oncol 14(8):769–776. doi:10.1016/S1470-2045(13)70162-1
Kerbel RS, Kamen BA (2004) The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer 4(6):423–436
Colleoni M, Rocca A, Sandri MT, Zorzino L, Masci G, Nole F, Peruzzotti G, Robertson C, Orlando L, Cinieri S, De Braud F, Viale G, Goldhirsch A (2002) Low-dose oral methotrexate and cyclophosphamide in metastatic breast cancer: antitumor activity and correlation with vascular endothelial growth factor levels. Ann Oncol 13 (1):73–80
Hamano Y, Sugimoto H, Soubasakos MA, Kieran M, Olsen BR, Lawler J, Sudhakar A, Kalluri R (2004) Thrombospondin-1 associated with tumor microenvironment contributes to low-dose cyclophosphamide-mediated endothelial cell apoptosis and tumor growth suppression. Cancer Res 64(5):1570–1574
Ghiringhelli F, Menard C, Puig P, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B (2007) Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56(5):641–648. doi:10.1007/s00262-006-0225-8
Di Lorenzo G, Montesarchio V, Autorino R, Bellelli T, Longo N, Imbimbo C, Morelli E, Giannarini G, Mirone V, De Placido S (2009) Phase 1/2 study of intravenous paclitaxel and oral cyclophosphamide in pretreated metastatic urothelial bladder cancer patients. Cancer 115(3):517–523. doi:10.1002/cncr.24055
Vaughn DJ, Srinivas S, Stadler WM, Pili R, Petrylak D, Sternberg CN, Smith DC, Ringuette S, de Wit E, Pautret V, George C (2009) Vinflunine in platinum-pretreated patients with locally advanced or metastatic urothelial carcinoma: results of a large phase 2 study. Cancer 115(18):4110–4117. doi:10.1002/cncr.24460
Pronzato P, Vigani A, Pensa F, Vanoli M, Tani F, Vaira F (1997) Second line chemotherapy with ifosfamide as outpatient treatment for advanced bladder cancer. Am J Clin Oncol 20(5):519–521
Bellmunt J, Theodore C, Demkov T, Komyakov B, Sengelov L, Daugaard G, Caty A, Carles J, Jagiello-Gruszfeld A, Karyakin O, Delgado FM, Hurteloup P, Winquist E, Morsli N, Salhi Y, Culine S, von der Maase H (2009) Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27(27):4454–4461. doi:10.1200/JCO.2008.20.5534
Witte RS, Elson P, Bono B, Knop R, Richardson RR, Dreicer R, Loehrer PJ (1997) Eastern Cooperative Oncology Group phase II trial of ifosfamide in the treatment of previously treated advanced urothelial carcinoma. J Clin Oncol 15(2):589–593
Mielke S, Sparreboom A, Mross K (2006) Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. Eur J Cancer 42(1):24–30. doi:10.1016/j.ejca.2005.06.030
Sonpavde G, Jian W, Liu H, Wu MF, Shen SS, Lerner SP (2009) Sunitinib malate is active against human urothelial carcinoma and enhances the activity of cisplatin in a preclinical model. Urol Oncol 27(4):391–399. doi:10.1016/j.urolonc.2008.03.017
Hahn NM, Stadler WM, Zon RT, Waterhouse D, Picus J, Nattam S, Johnson CS, Perkins SM, Waddell MJ, Sweeney CJ (2011) Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol 29(12):1525–1530. doi:10.1200/JCO.2010.31.6067
Srinivas S, Narayanan S, Harshman L, Lam A, Haas D, Poushnejad S, Pachynski R, Fan A, Vaishampayan U (2014) Phase II study of pazopanib with weekly paclitaxel in refractory urothelial cancer. J Clin Oncol 32(4s): abstr 299
Lamm D, Brausi M, O’Donnell MA, Witjes JA (2014) Interferon alfa in the treatment paradigm for non-muscle-invasive bladder cancer. Urol Oncol 32(1):35.e21–35.e30. doi:10.1016/j.urolonc.2013.02.010
Powles T, Vogelzang NJ, Fine GD, Eder JP, Braiteh FS, Loriot Y, Zambrano C, Bellmunt J, Burris HA, Teng SM, Shen X, Keoppen H, Hedge PS, Chen DS, Petrylak D (2014) Inhibition of PD-L by MPDL3280A and clinical activity in patients with metastatic urothelial cancer (UBC). J Clin Oncol 32(5s):abstr 5011
Bajorin DF, Dodd PM, Mazumdar M, Fazzari M, McCaffrey JA, Scher HI, Herr H, Higgins G, Boyle MG (1999) Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol 17(10):3173–3181
Bellmunt J, Choueiri TK, Fougeray R, Schutz FA, Salhi Y, Winquist E, Culine S, von der Maase H, Vaughn DJ, Rosenberg JE (2010) Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol 28(11):1850–1855. doi:10.1200/JCO.2009.25.4599
Acknowledgments
This study was supported by a Grant (HI12C1788030014, H14C1931010014) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Korea.
Ethical standard
This study was reviewed and approved by the Asan Medical Center Institutional Review Boards (IRB).
Conflict of interest
None to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Park, J.H., Lee, JL. Intravenous 3-weekly paclitaxel and metronomic oral cyclophosphamide in patients with advanced urothelial cancer previously treated with gemcitabine and platinum. Cancer Chemother Pharmacol 75, 247–254 (2015). https://doi.org/10.1007/s00280-014-2640-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2640-4