Abstract
Background
While gemcitabine/cisplatin (GC) is the gold standard regimen for patients with advanced urothelial carcinoma (aUC), either dose-reduced GC or gemcitabine/carboplatin (GCa) is an alternative option for “cisplatin-unfit” patients. However, few studies have compared outcomes with these commonly used regimens in the real-world setting.
Methods
We retrospectively reviewed patients with aUC who received full-dose GC, dose-reduced GC, or GCa as first-line salvage chemotherapy at two university hospitals between 2016 and 2020. Progression-free survival, cancer-specific survival, and overall survival, as well as best overall response and adverse event profiles, were compared among these three regimens.
Results
Of 105 patients, 41, 27, and 37 patients received full-dose GC, dose-reduced GC, and GCa, respectively. Significant differences were noted in the patients’ baseline age, primary site, and renal function among the three regimens. Sixty-nine (65.7%) patients died during a median follow-up period of 14 months. There was no significant difference among the three regimens for all survival outcomes and best overall response. However, the complete response rate of dose-reduced GC (2/27, 7.4%) appeared inferior to that of full-dose GC (9/41, 22.0%) or GCa (6/37, 16.2%). Regarding adverse event profiles, no significant difference was observed among the three regimens, except for significantly fewer cases with elevated alanine aminotransferase in the GCa group compared with the other groups.
Conclusions
This study compared the oncological and toxicological outcomes of full-dose GC, dose-reduced GC, and GCa in real-world patients with aUC. Unlike in the clinical trial setting, there were almost no significant differences among the three regimens.
Similar content being viewed by others
Background
For the last three decades, platinum-based chemotherapy has played a key role in the treatment of advanced urothelial carcinoma (aUC). Gemcitabine/cisplatin (GC) is currently the gold standard regimen for patients with aUC who are fit for cisplatin [1, 2], as well as dose-dense methotrexate/vinblastine/doxorubicin/cisplatin (ddMVAC) [3, 4]. However, 30–50% of patients with aUC are ineligible to receive cisplatin because of poor performance status, impaired renal function (creatinine clearance < 60 mL/min), hearing loss, peripheral neuropathy, and heart failure [5, 6]. For such “cisplatin-unfit” patients, gemcitabine/carboplatin (GCa), in which cisplatin in the GC regimen is replaced with another platinum-containing drug, carboplatin, can be an alternative [3, 4]. However, the efficacy of GCa was proven inferior to that of GC in a previous clinical trial [7]. The GC regimen with a reduced dosage of cisplatin (i.e., “dose-reduced GC”) can also be used for patients who are considered ineligible for “full-dose GC” for several reasons (typically, impaired renal function) in the real-world setting [8, 9]. However, few studies have compared the outcomes of these commonly used regimens (dose-reduced GC and GCa) [9]. Therefore, the present study aimed to investigate the oncological and toxicological outcomes of full-dose GC, dose-reduced GC, and GCa in real-world patients with aUC.
Patients and methods
Ethical approval and informed consent
The present study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board (IRB) of the Graduate School of Medicine and Faculty of Medicine, The University of Tokyo (approval number: 10565) and the IRB of Kyorin University School of Medicine (approval number: 1262). Given the retrospective nature of the study, the requirement for informed consent was waived by the IRB of the Graduate School of Medicine and Faculty of Medicine, The University of Tokyo and the IRB of Kyorin University School of Medicine.
Study population
We retrospectively reviewed 107 patients with aUC who received full-dose GC, dose-reduced GC, or GCa as first-line salvage chemotherapy at either The University of Tokyo Hospital or Kyorin University Hospital between January 2016 and August 2020. We excluded two patients owing to the lack of appropriate image evaluation after starting chemotherapy, leaving 105 patients for inclusion in the final analysis (The University of Tokyo Hospital, n = 35; Kyorin University Hospital, n = 70).
Treatment and assessment
The regimen choice for each patient (full-dose GC, dose-reduced GC, or GCa) was dependent on the attending physician’s decision. Generally, full-dose GC was used for patients with good performance status and normal renal function, while either dose-reduced GC or GCa was selected for those with poor performance status and/or impaired renal function. The full-dose GC regimen consisting of 1000 mg/m2 gemcitabine on days 1, 8, and 15 and 70 mg/m2 cisplatin on day 2 was given every 28 days. The cisplatin dosage in the dose-reduced GC regimen was determined at the physician’s discretion and ranged from 50 to 80%; however, most (22/27, 81%) patients received the 75% dosage. The GCa regimen consisting of 1000 mg/m2 gemcitabine on days 1 and 8 and area under the curve 5 carboplatin on day 1 was given every 21 days. All patients underwent evaluations every 1–3 months, which comprised routine blood tests and/or computed tomography. The treatment efficacy was assessed in accordance with the Response Evaluation Criteria in Solid Tumours (RECIST) v1.1 [10]. Adverse events were evaluated in accordance with the Common Terminology Criteria for Adverse events (CTCAE) v5.0 [11]. Estimated glomerular filtration rate (eGFR) was calculated using the revised formula for Japanese patients [12].
Endpoints and follow-up
As oncological endpoints, progression-free survival (PFS), cancer-specific survival (CSS), overall survival (OS), and best overall response according to the RECIST v1.1 criteria [10] were compared among the three regimens. As toxicological endpoints, comprehensive adverse event profiles, namely abnormal laboratory data, rash, fatigue, and vomiting, were also assessed in accordance with the CTCAE v5.0 criteria [11], as stated above. Follow-up started on the day of initiating first-line chemotherapy. Follow-up information was obtained as of September 2021.
Statistical analysis
Differences in patient characteristics among the three regimens were assessed using Student’s t-test or the χ2 test. Survival curves were generated using the Kaplan–Meier method, and the curves were compared using the log-rank test. A Cox proportional hazards regression model was used for univariate and multivariate analyses of PFS, CSS, and OS. All statistical analyses were performed using JMP Pro version 15.0.0 (SAS Institute, Cary, NC, USA), and P < 0.05 indicated a significant difference.
Results
Patient characteristics
The patients’ characteristics at the start of first-line chemotherapy are summarized in Table 1. Of the 105 patients, 41, 27, and 37 patients received full-dose GC, dose-reduced GC, and GCa, respectively. Patients treated with full-dose GC were younger, had less frequent upper urinary tract disease, and better renal function compared with the other groups. Fifteen of 41 (36.6%) patients in the full-dose GC group had a primary tumor in upper urinary tract or both, whereas 17 of 27 (63.0%) and 29 of 37 (78.4%) patients in the dose-reduced GC and GCa groups did so, respectively (P = 0.0040). Median eGFRs (mL/min/1.73 m2) were 68.1, 45.2, and 39.3, in the full-dose GC, dose-reduced GC, and GCa groups, respectively (P < 0.0001; note: two patients with missing data were excluded in the calculation of eGFR). There was no significant difference in the other parameters, namely Eastern Cooperative Oncology Group Performance Status (ECOG PS), resection of the primary site, prior neoadjuvant/adjuvant chemotherapy, and metastatic sites, among the three regimens. The median number of chemotherapy cycles was 3 (interquartile range [IQR], 2–4), 3 (IQR, 2–4), and 3 (IQR, 1.5–6.5), with full-dose GC, dose-reduced GC, and GCa, respectively (P = 0.26). During the study period after first-line chemotherapy, 25 (23.8%) patients received pembrolizumab as later-line treatment (full-dose GC, n = 11; dose-reduced GC, n = 9; GCa, n = 5).
Oncological outcomes
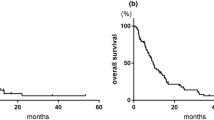
The median follow-up and survival times were 14 (IQR, 7–24) months and 18 (IQR, 8–32) months, respectively. Overall, 91 (86.7%) patients experienced disease progression, 64 (61.0%) died of aUC, and 5 (4.8%) died from other causes (pneumonia, n = 2; congestive heart failure, n = 1; myocarditis, n = 1; and gastric cancer, n = 1). There were no significant differences in PFS, CSS, and OS among the three regimens (Fig. 1).
Kaplan–Meier curves depicting A PFS, B CSS, and C OS of patients with aUC treated with full-dose GC, dose-reduced GC, or GCa. Abbreviations: aUC Advanced urothelial carcinoma, CSS Cancer-specific survival, GC Gemcitabine/cisplatin, GCa Gemcitabine/carboplatin, OS Overall survival, PFS Progression-free survival
Univariate analyses of PFS associated ECOG PS and resection of the primary site with the outcome, and multivariate analysis identified no resection of the primary site as an independent predictor of shorter PFS (Table 2). Univariate analyses of CSS associated ECOG PS, resection of the primary site, lymph node metastasis, and liver metastasis with the outcome, and multivariate analysis revealed ECOG PS ≥ 1, lymph node metastasis, and liver metastasis as independent predictors of shorter CSS (Table 3). Univariate and multivariate analyses of OS identified the same prognosticators as those identified for CSS (Table 4).
Figure 2 shows the best overall response results of the three regimens. Overall, 47 (44.8%) patients achieved an objective response (complete response [CR] + partial response [PR]). There were no significant differences in the objective response rate, durable response rate (CR + PR + stable disease), or progressive disease rate among the three regimens. Notably, the CR rate of dose-reduced GC (2/27, 7.4%) appeared inferior to that of full-dose GC (9/41, 22.0%) or GCa (6/37, 16.2%), although there was no significant difference (P = 0.28).
Toxicological outcomes
Figure 3 shows the comprehensive adverse event profiles among the three regimens, which were evaluated in 97 patients with sufficient data (full-dose GC, n = 41; dose-reduced GC, n = 24; GCa, n = 32). There were no significant differences in the rates of hematological toxicities (leukopenia, neutropenia, thrombocytopenia, and anemia), most hepatic toxicities (increased bilirubin and aspartate aminotransferase), renal toxicity (increased creatinine), rash, fatigue, and vomiting, among the three regimens. There were significantly fewer cases with increased alanine aminotransferase in the GCa group compared with the other groups (P = 0.025).
Comprehensive adverse event profiles among the three regimens (full-dose GC, dose-reduced GC, and GCa). The analysis was conducted for 97 patients with sufficient data (GCa, n = 32; full-dose GC, n = 41; dose-reduced GC, n = 24). Abbreviations: ALT alanine aminotransferase, AST aspartate aminotransferase, GC gemcitabine/cisplatin, GCa gemcitabine/carboplatin
Discussion
The present study compared the oncological and toxicological outcomes of full-dose GC, dose-reduced GC, and GCa in real-world patients with aUC. Unlike in the clinical trial setting, there were almost no significant differences in all endpoints assessed among the three regimens. Specifically, no significant difference was observed for all survival outcomes (PFS, CSS, and OS) and best overall response among the three regimens. Furthermore, the CR rate of dose-reduced GC (2/27, 7.4%) appeared inferior to that of full-dose GC (9/41, 22.0%) or GCa (6/37, 16.2%), albeit without a significant difference (P = 0.28). Similarly, no significant difference was observed for all but one toxicological endpoint among the three regimens, whereas there were significantly fewer cases with elevated alanine aminotransferase in patients undergoing GCa compared with the concentration in those receiving the other regimens (P = 0.025).
A randomized phase 2 trial comparing GC and GCa in “cisplatin-fit” patients (creatinine clearance ≥ 60 mL/min) reported an overall response rate of 49.1% for GC (CR: 14.5%; PR: 34.5%) and 40.0% for GCa (CR: 1.8%; PR: 38.2%). The authors also reported that median OS was 12.8 months and 9.8 months for GC and GCa, respectively, while no differences between the regimens were noted for the overall toxicity profiles [7]. Therefore, current guidelines do not recommend the use of GCa for “cisplatin-fit” patients with aUC [3, 4]. In contrast, another phase 2/3 trial comparing GCa and methotrexate/carboplatin/vinblastine (M-CAVI) in “cisplatin-unfit” patients (creatinine clearance 30–60 mL/min and/or ECOG PS 2) reported that the best overall response rates were 41.2% for GCa versus 30.3% for M-CAVI (P = 0.08) and that median OS was 9.3 months and 8.1 months for GCa and M-CAVI, respectively (P = 0.64). However, severe acute toxicity was observed in 9.3% of the patients receiving GCa and 21.2% of those receiving M-CAVI [13, 14]. Therefore, current guidelines recommend the use of GCa for “cisplatin-unfit” patients with aUC [3, 4]. Accordingly, the current European Association of Urology guideline classifies patients into the following three categories (with recommended first-line regimens): (1) fit for cisplatin (GC or ddMVAC is recommended); (2) unfit for cisplatin but fit for carboplatin (GCa is recommended); and (3) unfit for any platinum-based chemotherapy (immune checkpoint inhibitors, such as pembrolizumab and atezolizumab are considered) [3].
Our results may be inconsistent with those of the abovementioned clinical trials because the efficacy of GCa was comparable to that of GC, in our study. This difference is probably because of the retrospective design, small sample size, and selection bias. Nevertheless, given that GCa might have a higher CR rate than that of dose-reduced GC and that toxicities associated with GCa were similar (or lower) than those associated with dose-reduced GC, GCa might be preferable for “cisplatin-unfit” patients, rather than dose-reduced GC. The use of GCa could avoid toxicities induced by the long-term use of cisplatin, such as neurotoxicity, ototoxicity, and nephrotoxicity [15]. For reference, Ichioka et al. previously reported that the oncological outcomes of dose-reduced GC were inferior to those of full-dose GC in patients with eGFR < 60 mL/min/1.73 m2 [8]. More recently, Miyake et al. compared first-line full-dose GC, dose-reduced GC, and GCa in terms of the response to subsequent pembrolizumab treatment [9]. The authors reported that the response to pembrolizumab after dose-reduced GC was inferior to that after GCa in cisplatin-unfit patients with aUC and, thus, concluded that dose-reduced GC is not recommended for such patients [9].
Regarding the prognostic factors, poor performance status (ECOG PS ≥ 1) and liver metastasis were shown to be independent predictors of shorter CSS and OS (Tables 3, 4), in this study. These two factors have been reported as critical prognostic factors for patients with aUC [16,17,18], even in the era of immune checkpoint inhibitors [18]. Our results were similar to findings in the previous literature. In contrast, the type of first-line regimen (full-dose GC, dose-reduced GC, or GCa) was not associated with all of the survival endpoints that we assessed (PFS, CSS, and OS) even in the univariate analysis (Tables 2, 3, 4).
Although not assessed in the present study, gemcitabine plus split‑dose cisplatin (“GC split”) has been another well-known option for cisplatin-unfit patients with aUC [3, 19,20,21,22,23]. Several small-scale studies have evaluated “GC split” in UC patients with impaired renal function (creatinine clearance: 40–60 ml/min) using different split-dose schedules, all of which reported its feasibility and potential efficacy [19,20,21,22,23]. Nevertheless, given the study design of these previous studies (a small phase I/II trial or retrospective study), prospective randomized trials comparing “GC split” with conventional GC are warranted to validate this modified regimen [3].
The limitations of this study are the retrospective design, small sample size, and selection bias, as stated above. Additionally, owing to the multi-institutional retrospective study design, the dose reduction rate of cisplatin was not uniform. Nevertheless, this study might add additional evidence of the real-world outcomes of the commonly used regimens in patients eligible for platinum-based chemotherapy.
Conclusions
In real-world patients with aUC, there were almost no significant differences in both oncological and toxicological outcomes among patients receiving full-dose GC, dose-reduced GC, and GCa, unlike findings in the clinical trial setting.
Availability of data and materials
Because of ethical restrictions, the raw data underlying this study are available from the corresponding author upon reasonable request.
Abbreviations
- aUC:
-
Advanced urothelial carcinoma
- CR:
-
Complete response
- CSS:
-
Cancer-specific survival
- CTCAE:
-
Common Terminology Criteria for Adverse events
- ddMVAC:
-
Dose-dense methotrexate/vinblastine/doxorubicin/cisplatin
- ECOG PS:
-
Eastern Cooperative Oncology Group Performance Status
- eGFR:
-
Estimated glomerular filtration rate
- GC:
-
Gemcitabine/cisplatin
- GCa:
-
Gemcitabine/carboplatin
- IQR:
-
Interquartile range
- IRB:
-
Institutional Review Board
- M-CAVI:
-
Methotrexate/carboplatin/vinblastine
- OS:
-
Overall survival
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- RECIST:
-
Response evaluation criteria in solid tumours
References
von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77.
von der Maase H, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–8.
Cathomas R, Lorch A, Bruins HM, et al. EAU Muscle-invasive, Metastatic Bladder Cancer Guidelines Panel. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur Urol. 2022;81:95–103.
Flaig TW, Spiess PE, Agarwal N, et al. Bladder Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020;18:329–54.
Dash A, Galsky MD, Vickers AJ, et al. Impact of renal impairment on eligibility for adjuvant cisplatin-based chemotherapy in patients with urothelial carcinoma of the bladder. Cancer. 2006;107:506–13.
Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer “unfit” for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8.
Dogliotti L, Cartenì G, Siena S, et al. Gemcitabine plus cisplatin versus gemcitabine plus carboplatin as first-line chemotherapy in advanced transitional cell carcinoma of the urothelium: results of a randomized phase 2 trial. Eur Urol. 2007;52:134–41.
Ichioka D, Miyazaki J, Inoue T, et al. Impact of renal function of patients with advanced urothelial cancer on eligibility for first-line chemotherapy and treatment outcomes. Jpn J Clin Oncol. 2015;45:867–73.
Miyake M, Shimizu T, Nishimura N, et al. Response to pembrolizumab after dose-reduced cisplatin plus gemcitabine chemotherapy is inferior to that after carboplatin plus gemcitabine chemotherapy in cisplatin-unfit patients with advanced urothelial carcinoma. Clin Genitourin Cancer. 2022;20(196):e1-9.
Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50. Accessed 9 Apr 2022.
Matsuo S, Imai E, Horio M, et al. Collaborators developing the Japanese equation for estimated GFR. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis. 2009;53:982–92.
De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer “unfit” for cisplatin-based chemotherapy: phase II—results of EORTC study 30986. J Clin Oncol. 2009;27:5634–9.
De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol. 2012;30:191–9.
Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73:994–1007.
Taguchi S, Nakagawa T, Hattori M, et al. Prognostic factors for metastatic urothelial carcinoma undergoing cisplatin-based salvage chemotherapy. Jpn J Clin Oncol. 2013;43:923–8.
Taguchi S, Nakagawa T, Matsumoto A, et al. Pretreatment neutrophil-to-lymphocyte ratio as an independent predictor of survival in patients with metastatic urothelial carcinoma: a multi-institutional study. Int J Urol. 2015;22:638–43.
Taguchi S, Kawai T, Nakagawa T, et al. Prognostic significance of the albumin-to-globulin ratio for advanced urothelial carcinoma treated with pembrolizumab: a multicenter retrospective study. Sci Rep. 2021;11:15623.
Hussain SA, Stocken DD, Riley P, et al. A phase I/II study of gemcitabine and fractionated cisplatin in an outpatient setting using a 21-day schedule in patients with advanced and metastatic bladder cancer. Br J Cancer. 2004;91:844–9.
Hussain SA, Palmer DH, Lloyd B, et al. A study of split-dose cisplatin-based neo-adjuvant chemotherapy in muscle-invasive bladder cancer. Oncol Lett. 2012;3:855–9.
Morales-Barrera R, Bellmunt J, Suárez C, et al. Cisplatin and gemcitabine administered every two weeks in patients with locally advanced or metastatic urothelial carcinoma and impaired renal function. Eur J Cancer. 2012;48:1816–21.
Kim YR, Lee JL, You D, et al. Gemcitabine plus split-dose cisplatin could be a promising alternative to gemcitabine plus carboplatin for cisplatin-unfit patients with advanced urothelial carcinoma. Cancer Chemother Pharmacol. 2015;76:141–53.
Izumi K, Iwamoto H, Yaegashi H, et al. Gemcitabine plus cisplatin split versus gemcitabine plus carboplatin for advanced urothelial cancer with cisplatin-unfit renal function. In Vivo. 2019;33:167–72.
Acknowledgements
We thank Jane Charbonneau, DVM, from Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding
None.
Author information
Authors and Affiliations
Contributions
KS and ST contributed to the conception and design of the study, analysis and interpretation of the data, and drafted the first manuscript. KK, TK, KM, YN, MK, MT, JM, YA, YY, YS, and DY contributed to acquisition of the data. TN, HF, and HK supervised the study, helped draft the manuscript, and were involved in revising the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards, and was approved by the IRB of the Graduate School of Medicine and Faculty of Medicine, The University of Tokyo (Approval No.: 10565) and the IRB of Kyorin University School of Medicine (Approval No.: 1262). Because of the retrospective nature of the study, the requirement for informed consent was waived by the IRB of the Graduate School of Medicine and Faculty of Medicine, The University of Tokyo and the IRB of Kyorin University School of Medicine.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Sugimoto, K., Taguchi, S., Kishitani, K. et al. Comparison of full-dose gemcitabine/cisplatin, dose-reduced gemcitabine/cisplatin, and gemcitabine/carboplatin in real-world patients with advanced urothelial carcinoma. BMC Urol 22, 177 (2022). https://doi.org/10.1186/s12894-022-01139-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12894-022-01139-9