Abstract
Purpose
MNRP1685A is a human monoclonal antibody that blocks binding of vascular endothelial growth factor (VEGF), VEGF-B, and placental growth factor 2 to neuropilin-1 resulting in vessel immaturity and VEGF dependency. The safety of combining MNRP1685A with bevacizumab, with or without paclitaxel, was examined.
Methods
Patients with advanced solid tumors received escalating doses of MNRP1685A (7.5, 15, 24, and 36 mg/kg) with bevacizumab 15 mg/kg every 3 weeks in Arm A (n = 14). Arm B (n = 10) dosing consisted of MNRP1685A (12 and 16 mg/kg) with bevacizumab 10 mg/kg (every 2 weeks) and paclitaxel 90 mg/m2 (weekly, 3 of 4 weeks). Objectives were to determine safety, pharmacokinetics, pharmacodynamics, and the maximum tolerated dose of MNRP1685A.
Results
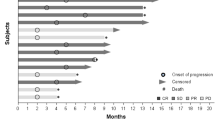
Infusion reactions (88 %) and transient thrombocytopenia (67 %) represent the most frequent study drug-related adverse events (AEs). Drug-related Grade 2 or 3 proteinuria occurred in 13 patients (54 %). Additional study drug-related AEs occurring in >20 % of patients included neutropenia, alopecia, dysphonia, fatigue, and nausea. Neutropenia occurred only in Arm B. Grade ≥3 study drug-related AEs in ≥3 patients included neutropenia (Arm B), proteinuria, and thrombocytopenia. Two confirmed and three unconfirmed partial responses were observed.
Conclusions
The safety profiles were consistent with the single-agent profiles of all study drugs. However, a higher than expected rate of clinically significant proteinuria was observed that does not support further testing of MNRP1685A in combination with bevacizumab.



Similar content being viewed by others
References
Tam SJ, Watts RJ (2010) Connecting vascular and nervous system development: angiogenesis and the blood-brain barrier. Annu Rev Neurosci 33:379–408
He Z, Tessier-Lavigne M (1997) Neuropilin is a receptor for the axonal chemorepellent semaphorin III. Cell 90:739–751
Soker S, Takashima S, Quan H et al (1998) Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell 92:735–745
Kawasaki T, Kitsukawa T, Bekku Y (1999) A requirement for neuropilin-1 in embryonic vessel formation. Development 126:4895–4902
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M et al (2002) Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem 277:24818–24825
Bielenberg DR, Pettaway CA, Takashima S et al (2006) Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res 312:584–593
Beck B, Driessens G, Goossens S et al (2011) A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumors. Nature 478:399–403
Grandclement C, Borg C (2011) Neuropilins: a new target for cancer therapy. Cancers 3:1899–1928
Liang W-C, Dennis MS, Stawicki S et al (2007) Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol 366:815–829
Xin Y, Bai S, Damico-Beyer LA et al (2012) Anti-neuropilin-1 (MRNP1685A): unexpected pharmacokinetic differences across species, from preclinical models to humans. Pharm Res 29:2512–2521
Xin Y, Li J, Wu J et al (2012) Pharmacokinetic and pharmacodynamic analysis of circulating biomarkers of anti-NRP1, a novel anti-angiogenesis agent, in two Phase I trials in patients with advanced solid tumors. Clin Cancer Res 18:6040–6048
Pan Q, Chanthery Y, Liang W-C (2007) Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell 11:53–67
Weekes CD1, Beeram M, Tolcher AW, Papadopoulos KP, Gore L, Hegde P, Xin Y, Yu R, Shih LM, Xiang H, Brachmann RK, Patnaik A (2014) A Phase I study of the human monoclonal anti-NRP1 antibody MNRP1685A in patients with advanced solid tumors. Invest New Drugs. doi:10.1007/s10637-014-0071-z
Motzer RJ, Michaelson MD, Redman BG et al (2006) Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol 24:16–24
Deprimo SE, Bello CL, Smeraglia J et al (2007) Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl Med 5:32
Darbonne WC, Uppal H, Du X, et al (2014) Mechanism for platelet reduction in anti-neuropilin-1 (MNRP1685A)-treated patients in patients with advanced solid tumors (submitted)
Gordon MS, Margolin K, Talpaz M et al (2001) Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol 19:843–850
Lu JF, Bruno R, Eppler S et al (2008) Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol 62:779–786
Liang W-C, Wu X, Peale FV et al (2006) Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J Biol Chem 2:951–961
Gerber H, Wu X, Yu L et al (2007) Mice expressing a humanized form of VEGF-A may provide insights into the safety and efficacy of anti-VEGF antibodies. PNAS 104:3478–3483
Robert B, Zhao X, Abrahamson DR (2000) Coexpression of neuropilin-1, Flk1, and VEGF(164) in developing and mature mouse kidney glomeruli. Am J Physiol Renal Physiol 279:F275–F282
Tapia R, Guan F, Gershin I et al (2008) Semaphorin3a disrupts podocyte foot processes causing acute proteinuria. Kidney Int 73:733–740
Acknowledgments
We thank the patients who participated in the study and their families. This study was sponsored by Genentech, Inc. Medical writing assistance was provided by Genentech, Inc.
Conflict of interest
AP: advisor to and research support from Genentech, Inc., PML: advisor to and research support from Genentech, Inc., WAM: research support from Genentech, Inc., KP: nothing to disclose, LG: nothing to disclose, MB: research support from Genentech, Inc., VR, AHK, JCB, LMS, WD, YX, RY, HX, RKB: employees of Genentech, Inc., and shareholders of F. Hoffmann La. Roche, Ltd., and CDW: advisor to and research support from Genentech, Inc. All authors had full control of all primary data and agree to allow the journal to review the data if requested.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Patnaik, A., LoRusso, P.M., Messersmith, W.A. et al. A Phase Ib study evaluating MNRP1685A, a fully human anti-NRP1 monoclonal antibody, in combination with bevacizumab and paclitaxel in patients with advanced solid tumors. Cancer Chemother Pharmacol 73, 951–960 (2014). https://doi.org/10.1007/s00280-014-2426-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2426-8