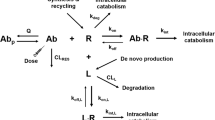
Abstract
Purpose
To compare the pharmacokinetics (PK) of MNRP1685A, a human monoclonal antibody (mAb) against neuropilin-1 (NRP1), in mice, rats, monkeys, and cancer patients from a Phase I study to model with parallel linear and nonlinear clearances.
Methods
Binding characteristics of MNRP1685A in different species were evaluated using surface plasmon resonance technology. PK profiles of MNRP1685A after single and/or multiple doses in different species were analyzed using population analysis. PK parameters were compared across species.
Results
MNRP1685A binds to NRP1 in all four species tested. Consistent with the wide expression of NRP1, MNRP1685A demonstrated pronounced non-linear PK over a wide dose range. PK profiles are best described by a two-compartment model with parallel linear and nonlinear clearances. Model-derived PK parameters suggest similar in-vivo target expression levels and binding affinity to target across all species tested. However, compared to typical human/humanized mAbs, non-specific clearance of MNRP1685A was faster in mice, rats, and humans (60.3, 19.4, and 8.5 ml/day/kg), but not in monkeys (3.22 ml/day/kg).
Conclusions
Monkey PK properly predicted the target-mediated clearance of MNRP1685A but underestimated its non-specific clearance in humans. This unique PK property warrants further investigation of underlying mechanisms.



Similar content being viewed by others
Abbreviations
- ATA:
-
anti-therapeutic antibody
- CHO cells:
-
Chinese hamster ovary cells
- CL:
-
non-specific clearance
- CLd :
-
distribution clearance
- CV:
-
coefficient of variation
- ELISA:
-
enzyme-linked immunosorbent assay
- FC:
-
flow cell
- FcRn:
-
neonatal Fc receptor
- HRP:
-
horseradish peroxidase
- IV:
-
intravenous
- KD :
-
equilibrium dissociation constant
- kg:
-
kilogram
- Km :
-
drug concentration at 50 % Vmax
- koff :
-
off-rate
- kon :
-
on-rate
- LLOQ:
-
lower limit of quantitation
- mAbs:
-
monoclonal antibodies
- mg:
-
milligram
- min:
-
minute
- ml:
-
milliliter
- mM:
-
millimolar
- ng:
-
nanogram
- nM:
-
nanomolar
- NRP1:
-
neuropilin-1
- PBS:
-
phosphate-buffered saline
- PK:
-
pharmacokinetics
- q3w:
-
every three weeks
- RSE:
-
relative standard error of estimation
- RU:
-
response unit
- SD:
-
standard deviation
- V1 :
-
apparent volume of central compartment
- V2 :
-
peripheral compartment distribution volume
- VEGF:
-
vascular endothelial growth factor
- Vmax :
-
maximum drug elimination by nonlinear (or specific) clearance
- μg:
-
microgram
- μl:
-
microliter
- σprop :
-
proportional residual error
- ωCL :
-
inter-subject variability on CL
- ωKm :
-
inter-subject variability on Km
- ωV1 :
-
inter-subject variability on V1
- ωVmax :
-
inter-subject variability on Vmax
References
Dirksand NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–59.
Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93:2645–68.
Mager DE. Target-mediated drug disposition and dynamics. Biochem Pharmacol. 2006;72:1–10.
Roskos LK, Davis CG, Schwab GM. The clinical pharmacology of therapeutic monoclonal antibodies. Drug Dev Res. 2004;61:108–20.
Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8.
Deng R, Iyer S, Theil FP, Mortensen DL, Fielder PJ, Prabhu S. Projecting human pharmacokinetics of therapeutic antibodies from nonclinical data: What have we learned? MAbs. 2010;3:61–6.
Ling J, Zhou H, Jiao Q, Davis HM. Interspecies scaling of therapeutic monoclonal antibodies: initial look. J Clin Pharmacol. 2009;49:1382–402.
Vugmeyster Y, Szklut P, Wensel D, Ross J, Xu X, Awwad M, Gill D, Tchistiakov L, Warner G. Complex pharmacokinetics of a humanized antibody against human amyloid Beta Peptide, anti-abeta ab2, in nonclinical species. Pharm Res. 2011;28:1696–706.
Bumbaca D, Wong A, Drake E, Reyes AE, 2nd, Lin BC, Stephan JP, Desnoyers L, Shen BQ, Dennis MS. Highly specific off-target binding identified and eliminated during the humanization of an antibody against FGF receptor 4. MAbs 2011;3:376–86..
Vugmeyster Y, Guay H, Szklut P, Qian MD, Jin M, Widom A, Spaulding V, Bennett F, Lowe L, Andreyeva T, Lowe D, Lane S, Thom G, Valge-Archer V, Gill D, Young D, Bloom L. In vitro potency, pharmacokinetic profiles, and pharmacological activity of optimized anti-IL-21R antibodies in a mouse model of lupus. MAbs. 2010;2:335–46.
Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–93.
Heand Z, Tessier-Lavigne M. Neuropilin is a receptor for the axonal chemorepellent Semaphorin III. Cell. 1997;90:739–51.
Kawasaki T, Kitsukawa T, Bekku Y, Matsuda Y, Sanbo M, Yagi T, Fujisawa H. A requirement for neuropilin-1 in embryonic vessel formation. Development. 1999;126:4895–902.
Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45.
Bernatchez PN, Rollin S, Soker S, Sirois MG. Relative effects of VEGF-A and VEGF-C on endothelial cell proliferation, migration and PAF synthesis: Role of neuropilin-1. J Cell Biochem. 2002;85:629–39.
Chen H, He Z, Bagri A, Tessier-Lavigne M. Semaphorin-neuropilin interactions underlying sympathetic axon responses to class III semaphorins. Neuron. 1998;21:1283–90.
Fujisawa H. From the discovery of neuropilin to the determination of its adhesion sites. Adv Exp Med Biol. 2002;515:1–12.
Mamluk R, Gechtman Z, Kutcher ME, Gasiunas N, Gallagher J, Klagsbrun M. Neuropilin-1 binds vascular endothelial growth factor 165, placenta growth factor-2, and heparin via its b1b2 domain. J Biol Chem. 2002;277:24818–25.
Cackowski FC, Xu L, Hu B, Cheng SY. Identification of two novel alternatively spliced Neuropilin-1 isoforms. Genomics. 2004;84:82–94.
Rossignol M, Gagnon ML, Klagsbrun M. Genomic organization of human neuropilin-1 and neuropilin-2 genes: identification and distribution of splice variants and soluble isoforms. Genomics. 2000;70:211–22.
Gagnon ML, Bielenberg DR, Gechtman Z, Miao HQ, Takashima S, Soker S, Klagsbrun M. Identification of a natural soluble neuropilin-1 that binds vascular endothelial growth factor: In vivo expression and antitumor activity. Proc Natl Acad Sci U S A. 2000;97:2573–8.
Lu Y, Xiang H, Liu P, Tong RR, Watts RJ, Koch AW, Sandoval WN, Damico LA, Wong WL, Meng YG. Identification of circulating neuropilin-1 and dose-dependent elevation following anti-neuropilin-1 antibody administration. MAbs. 2009;1:364–9.
Pan Q, Chathery Y, Wu Y, Rathore N, Tong RK, Peale F, Bagri A, Tessier-Lavigne M, Koch AW, Watts RJ. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J Biol Chem. 2007;282:24049–56.
Liang WC, Dennis MS, Stawicki S, Chanthery Y, Pan Q, Chen Y, Eigenbrot C, Yin J, Koch AW, Wu X, Ferrara N, Bagri A, Tessier-Lavigne M, Watts RJ, Wu Y. Function blocking antibodies to neuropilin-1 generated from a designed human synthetic antibody phage library. J Mol Biol. 2007;366:815–29.
Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, Ross S, Cheng Z, Le Couter J, Plowman G, Peale F, Koch AW, Wu Y, Bagri A, Tessier-Lavigne M, Watts RJ. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67.
Xin Y, Xiang H, Dresser M, Brachmann RK, Bai S. Characterization of MNRP1685A (anti-NRP1) Clinical Pharmacokinetics in a first-in-human Phase I Study. AAPS National Biotechnology Conference (2010).
Gibiansky L, Gibiansky E, Kakkar T, Ma P. Approximations of the target-mediated drug disposition model and identifiability of model parameters. J Pharmacokinet Pharmacodyn. 2008;35:573–91.
Dedrick RL. Animal scale-up. J Pharmacokinet Biopharm. 1973;1:435–61.
Lu JF, Bruno R, Eppler S, Novotny W, Lum B, Gaudreault J. Clinical pharmacokinetics of bevacizumab in patients with solid tumors. Cancer Chemother Pharmacol. 2008;62:779–86.
Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, Klein P. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56:361–9.
Ng CM, Lum BL, Gimenez V, Kelsey S, Allison D. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res. 2006;23:1275–84.
Bumbaca D, Xiang H, Boswell CA, Port RE, Stainton SL, Mundo EE, Ulufatu S, Bagri A, Theil FP, Fielder PJ, Khawli LA, Shen BQ. Maximizing tumor exposure to anti-neuropilin-1 antibody requires saturation of non-tumor tissue antigenic sinks in mice. Br J Pharmacol. 2012;166:368–77.
Ma P, Yang BB, Wang YM, Peterson M, Narayanan A, Sutjandra L, Rodriguez R, Chow A. Population pharmacokinetic analysis of panitumumab in patients with advanced solid tumors. J Clin Pharmacol. 2009;49:1142–56.
Kloft C, Graefe EU, Tanswell P, Scott AM, Hofheinz R, Amelsberg A, Karlsson MO. Population pharmacokinetics of sibrotuzumab, a novel therapeutic monoclonal antibody, in cancer patients. Invest New Drugs. 2004;22:39–52.
Hansel DE, Wilentz RE, Yeo CJ, Schulick RD, Montgomery E, Maitra A. Expression of neuropilin-1 in high-grade dysplasia, invasive cancer, and metastases of the human gastrointestinal tract. Am J Surg Pathol. 2004;28:347–56.
Darbonne WC, Du X, Dhawan P, Hartley D, Tarrant J, Taylor H, Cain G, Shih LM, Brachmann RK, Phung Q, Weekes CD, LoRusso P, Patnaik A, Xiang H, Ramakrishnan V. Mechanism for platelet reduction in anti-neuropilin-1 (MNRP1685A)–treated phase I patients. J Clin Oncol. 2011, 29: suppl; abstr e13598.
Acknowledgments and Disclosures
The authors acknowledge the valuable technical advice from Drs Rong Deng and Saileta Prabhu from Genentech Inc. in reviewing the work. The authors thank In Vivo Studies Group at Genentech for conducting the mouse and rat PK study, Derek Kennedy for coordinating the monkey studies, and Rashell Kinard for bioanalytic contributions. We would like to extend our thanks to Drs Christina de Zafra, Rodney Prell, Gary Cain, Ryan Watts, and Y. Gloria Meng for their contribution to the program.
Authors are employees at Genentech/Roche and hold Roche stocks.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
Goodness-of-fit model evaluation for mouse, rat, monkey, and human PK fittings. The open blue circles are observed concentrations. The black solid lines are lines of unity. The red solid lines are lines of linear regression. DV: dependent variable; PRED: population prediction from NONMEM analysis; IPRED; individual prediction from NONMEM analysis; WRES: weighted residuals for population in NONMEM analysis. (PPT 300 kb)
Rights and permissions
About this article
Cite this article
Xin, Y., Bai, S., Damico-Beyer, L.A. et al. Anti-Neuropilin-1 (MNRP1685A): Unexpected Pharmacokinetic Differences Across Species, from Preclinical Models to Humans. Pharm Res 29, 2512–2521 (2012). https://doi.org/10.1007/s11095-012-0781-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0781-x