Abstract
Purpose
Figitumumab (CP-751,871) is a human IgG2 monoclonal antibody that binds and down-regulates insulin-like growth factor receptor-1 (IGF-1R) and inhibits activation of this receptor by IGF-1 and IGF-2. This nonrandomized, open-label, single-arm, phase II trial evaluated the antitumor activity and safety of figitumumab in patients with metastatic colorectal cancer that was refractory to ≥2 systemic therapies.
Methods
Cohorts A and B received intravenous figitumumab 20 and 30 mg/kg in 3-week cycles, respectively. Both received loading doses (20 or 30 mg/kg) on days 1 and 2 of cycle 1. The primary endpoint was 6-month survival (null hypothesis for each cohort, H0: p6 mo surv = 0.45). Secondary endpoints included progression-free survival (PFS), overall survival (OS), objective response, safety, and pharmacokinetics.
Results
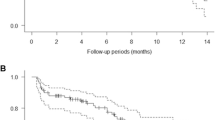
A total of 168 patients (Cohort A, n = 85; Cohort B, n = 83) received figitumumab. Estimated 6-month survival was 49.4 % (95 % CI 38.8–60.0) in Cohort A and 44.1 % (95 % CI 33.4–54.9) in Cohort B. Median OS was 5.8 and 5.6 months, respectively; median PFS was 1.4 months in both cohorts. No objective partial or complete responses occurred. The respective rates of treatment discontinuation due to treatment-related adverse events (AEs) were 5 and 7 %. The most common grade 3/4 nonhematologic AEs in both cohorts were hyperglycemia and asthenia. No grade 4 hematologic laboratory abnormalities occurred. Most deaths were reported as due to progressive disease; none were due to figitumumab.
Conclusion
Six-month survival data do not support further study of figitumumab 20 or 30 mg/kg in this patient population.


Similar content being viewed by others
References
Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127:2893–2917
Van Cutsem E, Köhne CH, Láng I, Folprecht G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Tejpar S, Schlichting M, Zubel A, Celik I, Rougier P, Ciardiello F (2013) Cetuximab plus irinotecan, fluorouracil, and leucovorin as first-line treatment for metastatic colorectal cancer: updated analysis of overall survival according to tumor KRAS and BRAF mutation status. J Clin Oncol 29:2011–2019
Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27:663–671
Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J (2010) Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol 28:4697–4705
Joulain F, Proskorovsky I, Allegra C, Tabernero J, Hoyle M, Iqbal SU, Van Cutsem E (2013) Mean overall survival gain with aflibercept plus FOLFIRI vs placebo plus FOLFIRI in patients with previously treated metastatic colorectal cancer. Br J Cancer 109:1735–1743
Shahda S, Saif MW (2013) Regorafenib: from bench to bedside in colorectal cancer. Expert Rev Clin Pharmacol 6:243–248
López-Calderero I, Sánchez Chávez E, García-Carbonero R (2010) The insulin-like growth factor pathway as a target for cancer therapy. Clin Transl Oncol 12:326–338
Donovan EA, Kummar S (2008) Role of insulin-like growth factor-1R system in colorectal carcinogenesis. Crit Rev Oncol Hematol 66:91–98
Cohen BD, Baker DA, Soderstrom C, Tkalcevic G, Rossi AM, Miller PE, Tengowski MW, Wang F, Gualberto A, Beebe JS, Moyer JD (2005) Combination therapy enhances the inhibition of tumor growth with the fully human anti-type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin Cancer Res 11:2063–2073
Haluska P, Shaw HM, Batzel GN, Yin D, Molina JR, Molife LR, Yap TA, Roberts ML, Sharma A, Gualberto A, Adjei AA, de Bono JS (2007) Phase I dose escalation study of the anti insulin-like growth factor-1 receptor monoclonal antibody CP-751,871 in patients with refractory solid tumors. Clin Cancer Res 13:5834–5840
Ii M, Li H, Adachi Y, Yamamoto H, Ohashi H, Taniguchi H, Arimura Y, Carbone DP, Imai K, Shinomura Y (2011) The efficacy of IGF-1 receptor monoclonal antibody against human gastrointestinal carcinomas is independent of k-ras status. Clin Cancer Res 17:5048–5049
National Cancer Institute (2012) Common Terminology Criteria for Adverse Events v3.0 (CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 23 March 2012
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Rao S, Cunningham D, de Gramont A, Scheithauer W, Smakal M, Humblet Y, Kourteva G, Iveson T, Andre T, Dostalova J, Illes A, Belly R, Perez-Ruixo JJ, Park YC, Palmer PA (2004) Phase III double-blind placebo-controlled study of farnesyl transferase inhibitor R115777 in patients with refractory advanced colorectal cancer. J Clin Oncol 22:3950–3957
Case L, Morgan T (2003) Design of Phase II cancer trials evaluating survival probabilities. BMC Med Res Methodol 3:6
Hart LS, Dolloff NG, Dicker DT, Koumenis C, Christensen JG, Grimberg A, El-Deiry WS (2011) Human colon cancer stem cells are enriched by insulin-like growth factor-1 and are sensitive to figitumumab. Cell Cycle 10:2331–2338
Yin D, Sleight B, Alvey C, Hansson AG, Bello A (2013) Pharmacokinetics and pharmacodynamics of figitumumab, a monoclonal antibody targeting the insulin-like growth factor 1 receptor, in healthy participants. J Clin Pharmacol 53:21–28
Juergens H, Daw NC, Geoerger B, Ferrari S, Villarroel M, Aerts I, Whelan J, Dirksen U, Hixon ML, Yin D, Wang T, Green S, Paccagnella L, Gualberto A (2011) Preliminary efficacy of the anti-insulin-like growth factor type 1 receptor antibody figitumumab in patients with refractory Ewing sarcoma. J Clin Oncol 29:4534–4540
Retraction (2012) Pre-treatment levels of circulating free IGF-1 identify NSCLC patients who derive clinical benefit from figitumumab. Br J Cancer 107:2024
Schmitz S, Kaminsky-Forrett MC, Henry S, Zanetta S, Geoffrois L, Bompas E, Moxhon A, Mignion L, Guigay J, Knoops L, Hamoir M, Machiels JP (2012) Phase II study of figitumumab in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: clinical activity and molecular response (GORTEC 2008-02). Ann Oncol 23:2153–2161
Chi KN, Gleave ME, Fazli L, Goldenberg SL, So A, Kollmannsberger C, Murray N, Tinker A, Pollak M (2012) A phase II pharmacodynamic study of preoperative figitumumab in patients with localized prostate cancer. Clin Cancer Res 18:3407–3413
Haluska P, Worden F, Olmos D, Yin D, Schteingart D, Batzel GN, Paccagnella ML, de Bono JS, Gualberto A, Hammer GD (2010) Safety, tolerability, and pharmacokinetics of the anti-IGF-1R monoclonal antibody figitumumab in patients with refractory adrenocortical carcinoma. Cancer Chemother Pharmacol 65:765–773
Acknowledgments
We would like to thank all of the participating patients and their families, as well as the global network of investigators, research nurses, study coordinators, and operations staff. This study was supported by funding from Pfizer Inc. Editorial assistance was provided by Susanne Gilbert at ACUMED® (New York, NY, USA) with funding from Pfizer Inc. Medical writing assistance was provided independently by Ann Morcos, MA, ELS, of US Oncology Research, McKesson Specialty Health (The Woodlands, TX, USA) with funding from the Texas Oncology-Sammons Cancer Center.
Conflict of interest
Carlos R. Becerra has received research funding from Pfizer Inc. Ramon Salazar has no conflicts to disclose. Rocio Garcia-Carbonero has served as an advisor/consultant to Pfizer, Merck, Amgen, and Roche. Anne L. Thomas has no conflicts to disclose. Federico J. Vázquez-Mazón has no conflicts to disclose. James Cassidy has received remuneration from Roche and has served as an advisor/consultant to Roche, Amgen, Sanofi, and GlaxoSmithKline. Tim Maughan has served as an advisor/consultant to Genentech and Sanofi. Manuel Gallén Castillo has no conflicts to disclose. Tim Iveson has no conflicts to disclose. Donghua Yin and Stephanie Green are employees of Pfizer Inc, and Stephanie Green holds stock in Pfizer. Emily K. Bergsland has served as an advisor/consultant to Pfizer (uncompensated).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Becerra, C.R., Salazar, R., Garcia-Carbonero, R. et al. Figitumumab in patients with refractory metastatic colorectal cancer previously treated with standard therapies: a nonrandomized, open-label, phase II trial. Cancer Chemother Pharmacol 73, 695–702 (2014). https://doi.org/10.1007/s00280-014-2391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2391-2