Abstract
Purpose
To compare the efficacy and toxicity of single-agent gemcitabine with gemcitabine plus cisplatin (G + C) in patients with metastatic pancreatic cancer
Methods
Forty-six patients with metastatic pancreatic cancer were randomized to receive gemcitabine alone (n = 25; 1,000 mg m−2) or G + C (n = 21; 1,000 mg m−2 gemcitabine and 25 mg m−2 cisplatin). Treatments were administered once a week for 3 weeks and repeated every 4 weeks.
Results
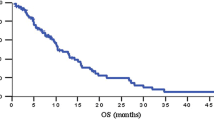
Patient characteristics were comparable between the gemcitabine alone and G + C groups. The gemcitabine dose intensity was similar between the gemcitabine alone and G + C groups (684 ± 32 vs. 617 ± 31 mg m−2 week−1). The cisplatin dose intensity was 15.1 ± 0.9 mg m−2 week−1 × 9.9 ± 1.8 weeks. Partial response rates were 8 % (2/25) for gemcitabine alone and 4.8 % (1/21) for G + C (p = 1). The median survival and median time to progression were 7.7 and 4.6 months for gemcitabine alone and 7.9 and 3.6 months for G + C, respectively (p = 0.752 and p = 0.857, respectively). Clinical benefit was 36 % for gemcitabine alone and 29 % for G + C (p = 0.592). Quality-adjusted life months were 5.6 ± 0.3 for the gemcitabine alone group and 3.8 ± 0.2 for the G + C group (p < 0.001). The frequency of grade 3/4 neutropenia (8 vs. 19 %) and anemia (8 vs. 10 %) and the number of hospitalization days per month of survival (4.7 ± 1.3 vs. 6.3 ± 1.6 days; p = 0.431) were not significantly different between patients who received gemcitabine alone and those who received G + C. However, patients in the G + C group had a higher rate of thrombocytopenia than did patients in the gemcitabine alone group (62 vs. 24 %; p = 0.009).
Conclusions
Gemcitabine alone and G + C had comparable and modest response rates in metastatic pancreatic cancer, but gemcitabine alone produced less toxicities than did G + C.


Similar content being viewed by others
References
Jemal A, Siegel R, Xu J et al (2010) (2010) Cancer statistics. CA Cancer J Clin 60(5):277–300
American Cancer Society. Survival rates for pancreatic cancer. http://www.cancer.org/Cancer/PancreaticCancer/OverviewGuide/pancreatic-cancer-overview-survival-rates
Burris HA 3rd, Moore MJ, Andersen J et al (1997) Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 15(6):2403–2413
Storniolo AM, Enas NH, Brown CA et al (1999) An investigational new drug treatment program for patients with gemcitabine: results for over 3,000 patients with pancreatic carcinoma. Cancer 85(6):1261–1268
Eastman A (1987) The formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. Pharmacol Ther 34(2):155–166
Plunkett W, Huang P, Searcy CE et al (1996) Gemcitabine: preclinical pharmacology and mechanisms of action. Semin Oncol 23(5 Suppl 10):3–15
Peters GJ, Ruiz van Haperen VW, Bergman AM et al (1996) Preclinical combination therapy with gemcitabine and mechanisms of resistance. Semin Oncol 23(5 Suppl 10):16–24
Greene FL, Page DL, Fleming ID et al (2002) Exocrine pancreas. In: AJCC cancer staging manual, 6th edn. Springer, New York, pp 157–164
Li CP, Chao Y, Chi KH et al (2003) Concurrent chemoradiotherapy treatment of locally advanced pancreatic cancer: gemcitabine versus 5-fluorouracil, a randomized controlled study. Int J Radiat Oncol Biol Phys 57(1):98–104
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst 92(3):205–216
Trotti A, Byhardt R, Stetz J et al (2000) Common toxicity criteria: version 2.0 an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47(1):13–47
Carmichael J, Fink U, Russell RC et al (1996) Phase II study of gemcitabine in patients with advanced pancreatic cancer. Br J Cancer 73(1):101–105
Casper ES, Green MR, Kelsen DP et al (1994) Phase II trial of gemcitabine (2,2′-difluorodeoxycytidine) in patients with adenocarcinoma of the pancreas. Invest New Drugs 12(1):29–34
Braakhuis BJ, van Ruiz Haperen VW, Welters MJ (1995) Schedule-dependent therapeutic efficacy of the combination of gemcitabine and cisplatin in head and neck cancer xenografts. Eur J Cancer 31A(13–14):2335–2340
Heinemann V, Wilke H, Mergenthaler HG et al (2000) Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol 11(11):1399–1403
Philip PA, Zalupski MM, Vaitkevicius VK et al (2001) Phase II study of gemcitabine and cisplatin in the treatment of patients with advanced pancreatic carcinoma. Cancer 92(3):569–577
Brodowicz T, Wolfram RM, Kostler WJ et al (2000) Phase II study of gemcitabine in combination with cisplatin in patients with locally advanced and/or metastatic pancreatic cancer. Anticancer Drugs 11(8):623–628
Colucci G, Giuliani F, Gebbia V et al (2002) Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer 94(4):902–910
Heinemann V, Quietzsch D, Gieseler F et al (2006) Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol 24(24):3946–3952
Cascinu S, Labianca R, Catalano V et al (2003) Weekly gemcitabine and cisplatin chemotherapy: a well-tolerated but ineffective chemotherapeutic regimen in advanced pancreatic cancer patients. A report from the Italian group for the study of digestive tract cancer (GISCAD). Ann Oncol 14(2):205–208
Hu J, Zhao G, Wang HX et al (2011) A meta-analysis of gemcitabine containing chemotherapy for locally advanced and metastatic pancreatic adenocarcinoma. J Hematol Oncol 4(1):11
de Xie R, Liang HL, Wang Y et al (2006) Meta-analysis of inoperable pancreatic cancer: gemcitabine combined with cisplatin versus gemcitabine alone. Chin J Dig Dis 7(1):49–54
Sultana A, Smith CT, Cunningham D et al (2007) Meta-analyses of chemotherapy for locally advanced and metastatic pancreatic cancer. J Clin Oncol 25(18):2607–2615
Park JK, Yoon YB, Kim YT et al (2008) Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol 42(1):86–91
Acknowledgments
This work was supported by grants from Taipei Veterans General Hospital (V101C-178), National Science Council (NSC 98-2314-B-075-029), and National Research Program for Biopharmaceutics of Taiwan (100CT202).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yee Chao and Chen-Yi Wu contributed equally to this manuscript.
Rights and permissions
About this article
Cite this article
Chao, Y., Wu, CY., Wang, J.P. et al. A randomized controlled trial of gemcitabine plus cisplatin versus gemcitabine alone in the treatment of metastatic pancreatic cancer. Cancer Chemother Pharmacol 72, 637–642 (2013). https://doi.org/10.1007/s00280-013-2239-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2239-1