Abstract
Purpose
Sorafenib and S-1 (one mixed formulation containing 5-FU prodrug and dihydropyrimidine dehydrogenase inhibitor) were two effective agents against hepatocellular carcinoma (HCC), but whether they had synergistic effects remained unclear. The present study aimed at evaluating their synergistic effects against HCC and its mechanisms.
Methods
Inhibitory effects of sorafenib, 5-FU and their combination on HCC cells PLC/PRF/5 and SK-HEP-1 were evaluated. Expressions of transcription factor E2F-1 and its downstream thymidylate synthetase (TS) in the treated cells were determined using real-time PCR and Western blot. In vivo anti-tumoral efficacy of S-1 plus sorafenib on HCC was evaluated in NOD/SCID mice. E2F-1 and TS expressions in tumors were determined by immunohistochemical staining.
Results
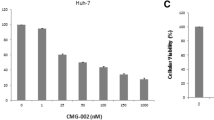
Sorafenib inhibited growth of HCC cells in dose-dependent manner, with IC50 of 5.4 ± 0.3 μmol/L for PLC/PRF/5 and 5.3 ± 0.5 μmol/L for SK-HEP-1. Sorafenib (1 μmol/L) enhanced inhibitory efficacy of 5-FU on HCC cells in vitro, dropping IC50 of 5-FU from 167.7 ± 12.1 to 105.4 ± 8.4 μmol/L for PLC/PRF/5 and 115 ± 10.2 to 82 ± 7.4 μmol/L for SK-HEP-1 (both p < 0.01). Sorafenib downregulated E2F-1 and TS expressions on HCC cells, and its combination with 5-FU yielded a synergistic downregulation of TS expression on HCC cells. In NOD/SCID mice with subcutaneously inoculated HCC, sorafenib combined with S-1 yielded greater inhibition on tumor growth and remarkable TS suppression when compared with sorafenib or S-1 alone (all p < 0.05).
Conclusions
Sorafenib enhanced therapeutic efficacy of 5-FU/S-1 against HCC through downregulation of E2F-1 and TS expressions. Sorafenib combined with S-1 might represent as valuable therapeutic regimen against HCC.






Similar content being viewed by others
References
Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, Wilhelm S, Lynch M, Carter C (2006) Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res 66:11851–11858
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J, SHARP Investigators Study Group (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359:378–390
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, Xu J, Sun Y, Liang H, Liu J, Wang J, Tak WY, Pan H, Burock K, Zou J, Voliotis D, Guan Z (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10:25–34
Ichikawa W, Uetake H, Shirota Y, Yamada H, Nishi N, Nihei Z, Sugihara K, Hirayama R (2003) Combination of dihydropyrimidine dehydrogenase and thymidylate synthase gene expressions in primary tumors as predictive parameters for the efficacy of fluoropyrimidine-based chemotherapy for metastatic colorectal cancer. Clin Cancer Res 9:786–791
Milano G, McLeod HL (2000) Can dihydropyrimidine dehydrogenase impact 5-fluorouracil-based treatment? Eur J Cancer 36:37–42
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, Fukushima M (1996) Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs 7:548–557
Shirasaka T, Shimamoto Y, Fukushima M (1993) Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res 53:4004–4009
Koizumi W, Kurihara M, Nakano S, Hasegawa K (2000) Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology 58:191–197
Shirao K, Ohtsu A, Takada H, Mitachi Y, Hirakawa K, Horikoshi N, Okamura T, Hirata K, Saitoh S, Isomoto H, Satoh A (2004) Phase II study of oral S-1 for treatment of metastatic colorectal carcinoma. Cancer 100:2355–2361
Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsui K, Kudoh S, Hasegawa K, Niitani H (2001) S-1 Cooperative Study Group (Lung Cancer Working Group), Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85:939–943
Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B (2001) Late phase II study of S-1 in patients with advanced head and neck cancer. Gan To Kagaku Ryoho 28:1381–1390
Okusaka T, Funakoshi A, Furuse J, Boku N, Yamao K, Ohkawa S, Saito H (2008) A late phase II study of S-1 for metastatic pancreatic cancer. Cancer Chemother Pharmacol 61:615–621
Furuse J, Okusaka T, Boku N, Ohkawa S, Sawaki A, Masumoto T, Funakoshi A (2008) S-1 monotherapy as first-line treatment in patients with advanced biliary tract cancer: a multicenter phase II study. Cancer Chemother Pharmacol 62:849–855
Naito S, Tsukamoto T, Usami M, Fujimoto H, Akaza H (2010) An early phase II trial of S-1 in Japanese patients with cytokine-refractory metastatic renal cell carcinoma. Cancer Chemother Pharmacol 66:1065–1070
Naito S, Eto M, Shinohara N, Tomita Y, Fujisawa M, Namiki M, Nishikido M, Usami M, Tsukamoto T, Akaza H (2010) Multicenter phase II trial of S-1 in patients with cytokine-refractory metastatic renal cell carcinoma. J Clin Oncol 28:5022–5029
Furuse J, Okusaka T, Kaneko S, Kudo M, Nakachi K, Ueno H, Yamashita T, Ueshima K (2010) Phase I/II study of the pharmacokinetics, safety and efficacy of S-1 in patients with advanced hepatocellular carcinoma. Cancer Sci 101:2606–2611
ChouTC Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Rustum YM (2004) Thymidylate synthase: a critical target in cancer therapy? Front Biosci 9:2467–2473
Copur S, Aiba K, Drake JC, Allegra CJ, Chu E (1995) Thymidylate synthase gene amplification in human colon cancer cell lines resistant to 5-fluorouracil. Biochem Pharmacol 49:1419–1426
Chu E, Voeller DM, Jones KL, Takechi T, Maley GF, Maley F, Segal S, Allegra CJ (1994) Identification of a thymidylate synthase ribonucleoprotein complex in human colon cancer cells. Mol Cell Biol 14:207–213
Chu E, Koeller DM, Johnston PG, Zinn S, Allegra CJ (1993) Regulation of thymidylate synthase in human colon cancer cells treated with 5-fluorouracil and interferon-gamma. Mol Pharmacol 43:527–533
Johnston PG, Drake JC, Trepel J, Allegra CJ (1992) Immunological quantitation of thymidylate synthase using the monoclonal antibody TS 106 in 5-fluorouracil-sensitive and -resistant human cancer cell lines. Cancer Res 52:4306–4312
Longley DB, Harkin DP, Johnston PG (2003) 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer 3:330–338
Ferguson PJ, Collins O, Dean NM, DeMoor J, Li CS, Vincent MD, Koropatnick J (1999) Antisense down-regulation of thymidylate synthase to suppress growth and enhance cytotoxicity of 5-FUdR, 5-FU and tomudex in HeLa cells. Br J Pharmacol 127:1777–1786
Yoshioka A, Tanaka S, Hiraoka O, Koyama Y, Hirota Y, Ayusawa D, Seno T, Garrett C, Wataya Y (1987) Deoxyribonucleoside triphosphate imbalance. 5-Fluorodeoxyuridine-induced DNA double strand breaks in mouse FM3A cells and the mechanism of cell death. J Biol Chem 262:8235–8241
Ayusawa D, Shimizu K, Koyama H, Takeishi K, Seno T (1983) Accumulation of DNA strand breaks during thymineless death in thymidylate synthase-negative mutants of mouse FM3A cells. J Biol Chem 258:12448–12454
Chu E, Drake JC, Koeller DM, Zinn S, Jamis-Dow CA, Yeh GC, Allegra CJ (1991) Induction of thymidylate synthase associated with multidrug resistance in human breast and colon cancer cell lines. Mol Pharmacol 39:136–143
DeGregori J, Kowalik T, Nevins JR (1995) Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol 15:4215–4224
Lin WC, Lin FT, Nevins JR (2001) Selective induction of E2F1 in response to DNA damage, mediated by ATM-dependent phosphorylation. Genes Dev 15:1833–1844
Chellappan SP, Hiebert S, Mudryj M, Horowitz JM, Nevins JR (1991) The E2F transcription factor is a cellular target for the RB protein. Cell 65:1053–1061
Drago-Ferrante R, Santulli A, Di Fiore R, Giuliano M, Calvaruso G, Tesoriere G, Vento R (2008) Low doses of paclitaxel potently induce apoptosis in human retinoblastoma Y79 cells by up-regulating E2F1. Int J Oncol 33:677–687
Komoto M, Nakata B, Nishii T, Kawajiri H, Shinto O, Amano R, Yamada N, Yashiro M, Hirakawa K (2010) In vitro and in vivo evidence that a combination of lapatinib plus S-1 is a promising treatment for pancreatic cancer. Cancer Sci 101:468–473
Tanizaki J, Okamoto I, Takezawa K, Tsukioka S, Uchida J, Kiniwa M, Fukuoka M, Nakagawa K (2010) Synergistic antitumor effect of S-1 and HER2-targeting agents in gastric cancer with HER2 amplification. Mol Cancer Ther 9:1198–1207
Okabe T, Okamoto I, Tsukioka S, Uchida J, Iwasa T, Yoshida T, Hatashita E, Yamada Y, Satoh T, Tamura K, Fukuoka M, Nakagawa K (2008) Synergistic antitumor effect of S-1 and the epidermal growth factor receptor inhibitor gefitinib in non-small cell lung cancer cell lines: role of gefitinib-induced down-regulation of thymidylate synthase. Mol Cancer Ther 7:599–606
Okabe T, Okamoto I, Tsukioka S, Uchida J, Hatashita E, Yamada Y, Yoshida T, Nishio K, Fukuoka M, Jänne PA, Nakagawa K (2009) Addition of S-1 to the epidermal growth factor receptor inhibitor gefitinib overcomes gefitinib resistance in non-small cell lung cancer cell lines with MET amplification. Clin Cancer Res 15:907–913
Takeuchi A, Shiota M, Tatsugami K, Yokomizo A, Eto M, Inokuchi J, Kuroiwa K, Kiyoshima K, Naito S (2011) Sorafenib augments cytotoxic effect of S-1 in vitro and in vivo through TS suppression. Cancer Chemother Pharmacol 68:1557–1564
Lee SJ, Lee J, Park SH, Park JO, Park YS, Kang WK, Lee J, Yim DS, Lim HY (2012) Phase I trial of S-1 in combination with sorafenib for patients with advanced hepatocellular carcinoma. Invest New Drugs 30:1540–1547
Acknowledgments
This work was supported by grants from National Natural Science Foundation of China (No. 30672051), Chinese Society of Clinical Oncology foundation (2010).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhai, JM., Yin, XY., Lai, YR. et al. Sorafenib enhances the chemotherapeutic efficacy of S-1 against hepatocellular carcinoma through downregulation of transcription factor E2F-1. Cancer Chemother Pharmacol 71, 1255–1264 (2013). https://doi.org/10.1007/s00280-013-2120-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-013-2120-2