Abstract
Purpose
Bosutinib is an orally active, dual Src/Abl tyrosine kinase inhibitor that has demonstrated manageable safety and high response rates in patients with chronic phase (CP) chronic myeloid leukemia (CML). The current analysis evaluated potential bosutinib pharmacokinetic–pharmacodynamic relationships.
Methods
Bosutinib exposure metrics at steady state were estimated from a previously developed population pharmacokinetic model. Safety and efficacy metrics were from two clinical studies of bosutinib 500 mg/day in patients with CP CML.
Results
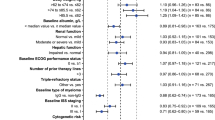
The analysis included 749 patients (aged 18–91 years; mean weight, 75 kg; 54 % male). An exposure–response relationship was identified for the pooled incidence (but not severity) of diarrhea, with predicted probability ranging from 0.575 to 0.797 for the lowest and highest area under the curve bins, respectively; a weak relationship was also observed for the incidence of rash (predicted probability, 0.216–0.419). There was no evidence of an exposure–response relationship for nausea, vomiting, neutropenia, thrombocytopenia, or elevated alanine and aspartate aminotransferases. Exposure–response relationships were observed in patients with newly diagnosed CP CML for complete cytogenetic response at 1 year (predicted probability, 0.476–0.650), major molecular response at 1 year (0.238–0.497), and cumulative complete hematologic response (CHR) at 1 year (0.605–0.763). Patients with previously treated CP CML showed no exposure–response relationship for major cytogenetic response at 24 weeks (0.320); for CHR, higher bosutinib exposure was associated with a lower probability of response (0.926–0.743).
Conclusions
The absence of exposure–response relationships for some safety and efficacy metrics may reflect bosutinib exposure metrics that exceeded the half-maximal inhibitory values and achieved a maximum effect.



Similar content being viewed by others
References
Puttini M, Coluccia AM, Boschelli F, Cleris L, Marchesi E, Donella-Deana A, Ahmed S, Redaelli S, Piazza R, Magistroni V, Andreoni F, Scapozza L et al (2006) In vitro and in vivo activity of SKI-606, a novel Src-Abl inhibitor, against imatinib-resistant Bcr-Abl +neoplastic cells. Cancer Res 66:11314–11322
Golas JM, Arndt K, Etienne C, Lucas J, Nardin D, Gibbons J, Frost P, Ye F, Boschelli DH, Boschelli F (2003) SKI-606, a 4-anilino-3-quinolinecarbonitrile dual inhibitor of Src and Abl kinases, is a potent antiproliferative agent against chronic myelogenous leukemia cells in culture and causes regression of K562 xenografts in nude mice. Cancer Res 63:375–381
Daud AI, Krishnamurthi SS, Saleh MN, Gitlitz BJ, Borad MJ, Gold PJ, Chiorean EG, Springett GM, Abbas R, Agarwal S, Bardy-Bouxin N, Hsyu PH et al (2012) Phase I study of bosutinib, a Src/Abl tyrosine kinase inhibitor, administered to patients with advanced solid tumors. Clin Cancer Res 18:1092–1100
Cortes JE, Kantarjian HM, Brummendorf TH, Kim D-W, Turkina AG, Shen Z-X, Pasquini R, Khoury HJ, Arkin S, Volkert A, Besson N, Abbas R et al (2011) Safety and efficacy of bosutinib (SKI-606) in chronic phase Philadelphia chromosome-positive chronic myeloid leukemia patients with resistance or intolerance to imatinib. Blood 118:4567–4576
Khoury HJ, Cortes JE, Kantarjian HM, Gambacorti-Passerini CB, Baccarani M, Kim DW, Zaritskey A, Countouriotis A, Besson N, Leip E, Kelly V, Brummendorf TH (2012) Bosutinib is active in chronic phase chronic myeloid leukemia after imatinib and dasatinib and/or nilotinib therapy failure. Blood 119:3403–3412
Cortes JE, Kim Dong, Kantarjian HM, Brummendorf TH, Dyagil I, Griskevicus L, Malhotra H, Powell C, Gogat K, Countouriotis AM, Gambacorti-Passerini C (2012) Bosutinib versus imatinib in newly diagnosed chronic phase chronic myeloid leukemia: results from the BELA trial. J Clin Oncol (in press)
Hsyu P-H, Mould D, Abbas R, Pearce S, Amantea M (2011) A population pharmacokinetic model of bosutinib. Poster presented at: the AACR-EORTC-NCI molecular targets and cancer therapeutics conference; November 12–16, 2011; San Francisco, CA 2011. Abstract A195
Campone M, Bondarenko I, Brincat S, Hotko Y, Munster PN, Chmielowska E, Fumoleau P, Ward R, Bardy-Bouxin N, Leip E, Turnbull K, Zacharchuk C et al (2012) Phase II study of single-agent bosutinib, a Src/Abl tyrosine kinase inhibitor, in patients with locally advanced or metastatic breast cancer pretreated with chemotherapy. Ann Oncol 23:610–617
Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoli J, Paquette R, Cortes J, O’Brien S, Nicaise C, Bleickardt E, Blackwood-Chirchir MA (2006) Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 354:2531–2541
Hochhaus A, Kantarjian HM, Baccarani M, Lipton JH, Apperley JF, Druker BJ, Facon T, Goldberg SL, Cervantes F, Niederwieser D, Silver RT, Stone RM et al (2007) Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood 109:2303–2309
Kantarjian H, Pasquini R, Hamerschlak N, Rousselot P, Holowiecki J, Jootar S, Robak T, Khoroshko N, Masszi T, Skotnicki A, Hellmann A, Zaritsky A et al (2007) Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood 109:5143–5150
Shah NP, Kantarjian HM, Kim DW, Rea D, Dorlhiac-Llacer PE, Milone JH, Vela-Ojeda J, Silver RT, Khoury HJ, Charbonnier A, Khoroshko N, Paquette RL et al (2008) Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol 26:3204–3212
Kantarjian H, Giles F, Wunderle L, Bhalla K, O’Brien S, Wassmann B, Tanaka C, Manley P, Rae P, Mietlowski W, Bochinski K, Hochhaus A et al (2006) Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Engl J Med 354:2542–2551
Kantarjian HM, Giles F, Gattermann N, Bhalla K, Alimena G, Palandri F, Ossenkoppele GJ, Nicolini FE, O’Brien SG, Litzow M, Bhatia R, Cervantes F et al (2007) Nilotinib (formerly AMN107), a highly selective BCR-ABL tyrosine kinase inhibitor, is effective in patients with Philadelphia chromosome-positive chronic myelogenous leukemia in chronic phase following imatinib resistance and intolerance. Blood 110:3540–3546
Quintas-Cardama A, Kantarjian H, Jones D, Nicaise C, O’Brien S, Giles F, Talpaz M, Cortes J (2007) Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood 109:497–499
Garg RJ, Kantarjian H, O’Brien S, Quintas-Cardama A, Faderl S, Estrov Z, Cortes J (2009) The use of nilotinib or dasatinib after failure to 2 prior tyrosine kinase inhibitors: long-term follow-up. Blood 114:4361–4368
Giles FJ, Abruzzese E, Rosti G, Kim DW, Bhatia R, Bosly A, Goldberg S, Kam GL, Jagasia M, Mendrek W, Fischer T, Facon T (2010) Nilotinib is active in chronic and accelerated phase chronic myeloid leukemia following failure of imatinib and dasatinib therapy. Leukemia 24:1299–1301
Acknowledgments
Clinical studies 3160A4-200-WW and 3160A4-3000-WW were sponsored by Wyeth Research, which was acquired by Pfizer Inc in October 2009; the current pharmacokinetic–pharmacodynamic analysis was sponsored by Pfizer Inc. Poe-Hirr Hsyu and Michael Amantea are employees of and own stock in Pfizer. Diane Mould and Richard Upton are employees of Projections Research Inc and served as consultants/advisors to Pfizer through their employment. Medical writing support was provided by Janetricks N. Chebukati, PhD, of SciFluent and was funded by Pfizer Inc.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hsyu, PH., Mould, D.R., Upton, R.N. et al. Pharmacokinetic–pharmacodynamic relationship of bosutinib in patients with chronic phase chronic myeloid leukemia. Cancer Chemother Pharmacol 71, 209–218 (2013). https://doi.org/10.1007/s00280-012-1998-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-012-1998-4